Introduction
Restorative Dentistry has irrevocably entered a revolutionary era of aesthetic restorative materials. Patients’ demand for aesthetic restorations & the search for filling materials that can provide long-term stability are leading to development of new products in adhesive dentistry at an unprecedented rate[1].
The foundation of modern adhesive dentistry was laid in 1955 when Buonocore reported that acids could be used to alter the surface of enamel to render it more receptive to adhesion[2]. Acid etching of the enamel with phosphoric acid produced micro porosities in the enamel allowing resin bonding via micro-mechanical retention[3].
Acid etching of the enamel gave way to total etch techniques, in which both the enamel and dentin surfaces are acid conditioned to allow for resin adherence to both enamel and dentin surfaces[3]. Bonding to enamel is more reliable whereas bonding to dentin represents a greater challenge and has proved to be more difficult and less predictable. Difficulties in bonding to dentin are a result of inherent characteristics of this substrate (Perdiago, 2002; Lopes et al, 2002; Perdiago, Lopes, 1999)[4]. While enamel is 92% inorganic hydroxyapatite by volume, dentin is about 45% inorganic & the rest being organic & water.
To counteract these problems constant active research in the field of adhesive dentistry has resulted in the rapid evolution of dental adhesive systems through several generations with changes in chemistry, mechanisms, number of bottles, application techniques & clinical effectiveness.
Adhesive dentistry involves two methods: the total-etch bonding technique & Self-etching systems[4]. Dentin Adhesives are currently available as two step, and single step systems depending on how the three cardinal steps of etching, priming and bonding to tooth substrate are accomplished or simplified. Two step systems are sub-divided into the self priming adhesives that require a separate etching step, and the self etching primers that require an additional bonding step. The recently introduced all in one adhesives further combined these three bonding procedures into a single step application[5].
The current concept has proved itself both scientifically and clinically .The concept reduces the clinical steps, can be placed inexpensively, provides adequate bonding to enamel and dentin and most importantly, ensures the patients post operative comfort[6].
The purpose of this in-vitro study was to comparatively evaluate the shear bond strength of these newer dentin bonding agents i.e. two-step etch & rinse adhesives i.e. Prime & Bond NT and one-step self etching agents i.e. G Bond, Xeno V & two step self-etching adhesive i.e. Adper SE Plus.
Materials and Method
Materials used in the study: (Figure 1)
Dentin Bonding Agents used:
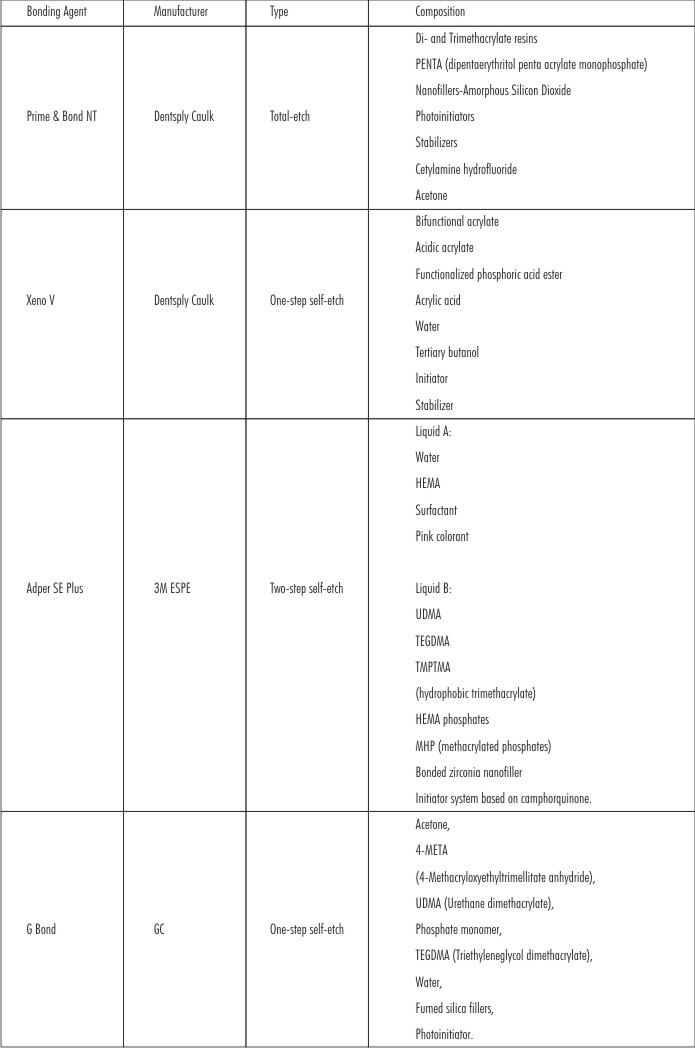 | Materials used in the study:Dentin Bonding Agents used:
 |
 | Figure 1: Materials used for specimen preparation, Bonding Agents, A : 37% phosphoric acid and Prime & Bond NT, B : Xeno V, C : Adper SE Plus, D : G-Bond, Composite Material, E : Filtek Z350 XT
 |
Operative Procedure
Sample collection, cleaning and storage:
Eighty freshly extracted non-carious intact human molars were selected for this study. Teeth with restorations, cracks, attrition and other structural defects were excluded. After extraction all the teeth collected were cleaned of blood and saliva in running water. Calculus was removed with the help of a scaler followed by thorough cleaning with pumice slurry and rubber prophylaxis cup. The teeth were then stored in normal saline solution. These specimen teeth were utilized for this study within six months of extraction as per ISO standards (G. Oilo, 1993).
Preparation and grouping of the specimens for shear bond strength:
To standardize depth of cavity, depth holes were drilled in the deepest part of central fossa of each tooth. The occlusal surfaces of all the teeth were ground on water-cooled orthodontic trimmer to obtain a flat dentinal surface.
Steel moulds of dimension 2.1 x 2.1 cm were placed in position over the teeth. A thin mix of auto-polymerizing acrylic resin was poured in the moulds to embed the prepared tooth, exposing the crown portion of the tooth (Figure 2). The prepared specimens were then randomly divided into four groups, based on dentin bonding agent used, with twenty specimens in each group, namely:
Group I – for Prime & Bond NT
Group II – for Xeno V
Group III – for Adper SE Plus
Group IV – for G Bond
The mounted teeth were then stored in normal saline at room temperature.
 | Figure 2: Chrome Plated Steel mould for mounting of tooth specimen
 |
Application of bonding agent and composite build up:-
Bonding agent (Prime&Bond NT, G Bond, Xeno V, and Adper SE Plus) was applied on the flat dentinal surface of each mounted tooth of all the four groups according to the manufacturers’ instructions.
The split-teflon mould was placed over the bonded surface at the pre-designed location in the mould holder. Filtek Z350 XT (3M ESPE) was placed in increments into the circular punch hole measuring 3 mm × 3 mm in a Teflon sheet and cured layer by layer for 20 seconds with SmartLite PS (Dentsply) (intensity >500 mW/cm2) (Figure 3 a and b).
Once the composite was cured, the split teflon mould was gently lifted out and specimens were obtained.
 | Figure 3 a: Chrome plated steel mold holder and Split Teflon mould used in shear bond strength study
 |
 | Figure 3 b: Chrome plated steel mold holder and Split Teflon mould with tooth specimen in place for composite build-up
 |
Thermocycling of the specimens:-
Specimens were then stored in distilled water at 37I0;C for 24 hours. The thermocycling of specimens of each group was carried out in accordance with the ISO standards that are 500 cycles at 5ºC and 55ºC, dwell time of 30 seconds and transfer time of 15 seconds (G.Oilo, 1993).
Shear bond strength testing:-
Specimens were then mounted on a Universal Testing Machine in the Department of Textile Engineering, Indian Institute of Technology (IIT), Delhi and loaded until resin-dentin interface fractured. The machine was adjusted to operate on a load of 100 Kgs. A cross-head speed of 1 mm/minute was used and the breaking load was measured by recording the reading on the display panel of the machine. The breaking load in kilograms was then converted into bond strength in megapascals.
For shear bond strength testing, the steel moulds with the prepared specimens adapted in them, were adjusted vertically in the inferior (static) jaw of the Universal Testing Machine. The placement of the specimen in the steel mould was such that the composite build up stood parallel to the floor. A key with dimensions same as that of the composite cylinder (3 mm x 3 mm), gripped in the superior jaw of the Instron machine, was adapted on to the composite build up. The shear force was then applied at the resin-dentin interface until the dislodgement of the composite cylinder from the dentinal surface occurred (Figure 4).
 | Figure 4: Specimens positioned in INSTRON for shear testing.
 |
The results of all four groups were then statistically analysed using SPSS 10 software for one-way ANOVA tests followed by Tukeys HSD test to determine if significant differences existed between the groups.
Results
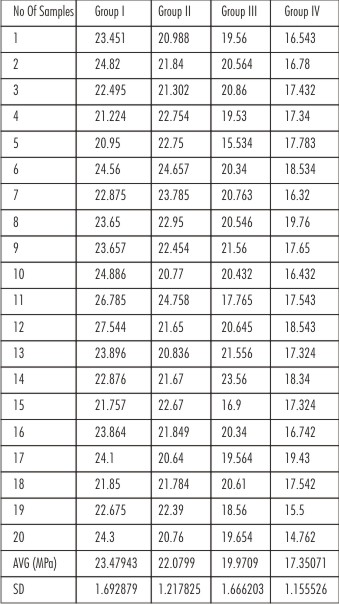
Shear bond strengths (MPa) of each specimen of all four groups were obtained (Table 1), and then put to statistical analysis.
The results revealed that samples of Group I (Prime & Bond NT) had the maximum shear bond strength (23.479 MPa).
Strength decreasing order: Group I > Group II > Group III > Group IV
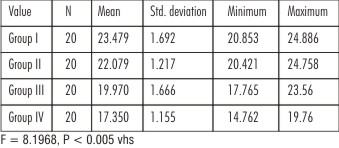
When a comparison of the shear bond strength of total etch and newer self etch adhesives was made using one-way ANOVA, it showed high statistically significant results. (Table 2)
p < 0.005. So our experimental results will give 0.05% error or 95% confident level at significance level. Hence, multigroup comparison was done using Tukeys HSD Test.
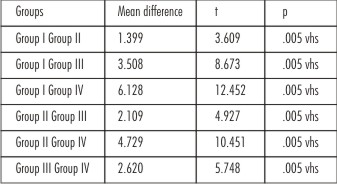
The shear bond strength values of the control group Prime & Bond NT (Group I) showed the highest mean bond strength values compared to the experimental groups of self etch adhesives (Group II, Group III and Group IV). Intergroup comparison was done between the adhesives using Tukey HSD test. The shear bond strength values of Group I (Prime & Bond NT) versus Group II (Xeno V), Group III (Adper SE Plus), Group IV (G-Bond), and showed high significance level. This indicated that the total etch adhesives have better bonding ability as compared to the newer self etching adhesives. (Table 3)
Intercomparison was done between the self etch adhesives using Tukeys HSD test. Comparison between Group II (Xeno V) and Group III (Adper SE Plus) also showed statistical significant difference (p<0.05) indicating that one-step self etch adhesive Xeno V is a better dentin bonding agent than two-step self etch adhesive Adper SE Plus.
Comparison of Group II (Xeno V) with Group IV (G-Bond), which are both one-step self-etch adhesives, showed high statistically significant difference inferring that Xeno V had comparatively higher bond strength to dentin.
When intergroup comparison was done between Group III and Group IV using Tukeys HSD test, the results proved to be significant statistically, showing that Adper SE Plus had better bond strength than G bond.
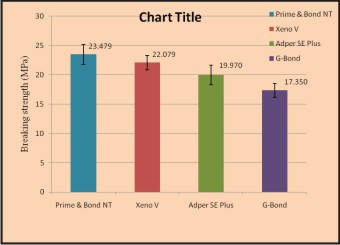
Conclusion: Among these four groups, Group I had highest shear bond strength (Avg 23.48 MPa), and Group IV had minimum shear bond strength (Avg 17.35 MPa) (Figure 5).
 | Table 1. Shear Bond Strength in Megapascals (MPa)
 |
 | Table 2: Mean and standard deviation values for shear bond strength
 |
 | Table 3: Intergroup comparison of shear bond strength values
 |
 | Figure 5. Bar Diagram Showing Comparison of Mean Shear Bond Strengths in MPa of various Study Groups
 |
Discussion
Ever since the introduction of dental composite resins by Bowen (1962) and the concept of retention using acid etching by Buonocore (1955), there has been extensive addition to their plethora of applications. Dental composites with dentine adhesive systems are currently the most popular materials for restorations.
Over the last three decades, numerous dentin adhesive systems have been released in the market, all claiming their own specific adhesion strategy. Clinicians are faced with many challenges when selecting a dental adhesive system to bond resin-based materials to mineralized tooth structure. The current trend in adhesive dentistry is focused on using systems that are simple to use and have minimal chair-side time[7].
Modern dentin adhesive systems generally use one of two adhesion strategies based on method of dealing with the smear layer [8]. One strategy is to modify and infiltrate the smear layer with resin, while the other is based on a so-called hybridization process in which complete removal of the smear layer and concurrent demineralization of the dentin surface layer, followed by resin interdiffusion into the microporosities of the exposed dentinal collagen matrix [9].
Shear type of test is the most commonly used methods as described by the ISO Standards [10]. Barkmeier and Cooley (1992) said that the shear bond strength test is a simple evaluation procedure used to test the adhesion of dental adhesives. In vitro bond strength tests are useful and essential for predicting the performance of adhesive systems and have a correlation with clinical issues [4],[11].
The present in-vitro study compared in vitro shear bond strength of the 5th generation total-etch dentin adhesive Prime and Bond NT with newer self-etch bonding systems Adper SE Plus, Xeno V, and G bond.
Thermal cycling allows bonded specimens to be subjected to extreme temperatures, which mimic intraoral temperature variations (M Miya Zaki et al, 2000)[12]. So in the present study, thermocycling of specimens of each group was carried out in accordance with the ISO standards i.e. 500 cycles at 5ºC and 55ºC, dwell time of 30 seconds and transfer time of 15 seconds[13].
Shear bond strength testing was done with a Universal Testing Machine, Instron, (Instron - UTM, Model 4202). Instron machine is conventionally popular for evaluating the adhesive ability of adhesive /restorative materials. With the simple technique and relevant results it is considered a benefit for ranking and marketing purposes[14], [15]. So in the present study, the machine was adjusted to operate on a load range of 0-100 kilograms. ISO recommended values of cross-head speed i.e. 0.45-1.05 mm/minute were taken in to consideration and 1 mm/minute cross head speed was used.
Kiremitci A. et al (2004) postulated that minimum bond strength of 17-20 MPA to enamel and dentin is needed to resist contraction forces of resin composite materials [16]. Clinical experiences confirm this bond strength is sufficient for successful retention of resin restoration.[17]
All adhesive systems used in this present study - total etch adhesive Prime & Bond NT (Group I); two-step self-etch adhesive system Adper SE Plus (Group III) and one-step self-etch adhesive systems Xeno V (Group II) and G-Bond (Group IV), achieved the optimal bond strength values for dentin. However, the total etch system Prime & Bond NT showed better bond strength compared to the self etching adhesives - Xeno V, Adper SE Plus and G-Bond.
This result was in accordance with Bouillaguet et al, Chuang et al, Kerby et al who concluded that self etching adhesives have lower bond strength as compared to total etch bonding systems [18], [19], [20]. Senawongse et al also demonstrated that 2 self etching systems, One – up bond and Clearfil SE bond, had lower bond strength than did the total etch system Single bond.[14] According to Hashimato et al, self etch adhesives produced thinner and shorter resin tags than those produced by phosphoric acid etching and thus resulted in inferior bond strength as compared to total etch adhesive systems.[20], [21]
The highest shear bond strength of Prime & Bond NT (Group I) when compared with the various other dentin bonding agents used (Group II, Group III & Group IV) can be explained by the fact that PENTA in Prime & Bond NT is a molecule of mild acidity that behaves as a conditioning agent (Perdigao et al, 1994)[22]. PENTA is claimed to bond ionically to dentinal calcium [22] and is an adhesion promoter that facilitates the penetration of resin monomer into the dentin for micromechanical bonding. UDMA in Prime & Bond NT is a hydrophobic monomer for proper polymerization and cross linking and bonds to surface-bound hydroxyl groups through its urethane groups (Andre V. Ritter et al, 2000)[23]. This could have resulted in better penetration through smear layer and improved contact of monomer within the exposed collagen fibres, resulting in a homogenous interface with no voids and hence, better bonding.
According to the present study self etch adhesives showed lower bond strength as compared to total etch bonding systems. Self etching adhesives provide lower bond strength than total etch systems because of their semi permeability, incorporation of smear layer, shorter resin tag formation, residual acidity and hydrolytic instability[24].The phosphoric acid etching and water rinsing in total-etch to remove dissolved calcium phosphate would prevent entrapment of this residue within the bonding resin layer which might otherwise interfere with resin penetration as seen in self etching adhesives[25].
Self etching adhesive systems rely on acidic monomers to simultaneously demineralize and infiltrate enamel and dentin. This acidity must be neutralized by the mineral content of the tooth structure to allow complete polymerization of the adhesive film [26]. With total-etch adhesive, smear layer and dissolved mineral are removed during the rinsing step. But because of some questions regarding residual acidity of the self-etch adhesives, and the fact that the smear layer is not removed, the issue of long term hydrolytic stability of the self etching adhesive systems still remains unresolved [3], [27]. Further the presence of smear layer in self-etch adhesives could have inhibited the penetration of the primer/resin into collagen fibres[28].
However, Kiremitci et al (2004) concluded that self etching adhesive system produced higher bond strength than conventional total etch systems, especially all in one system which produced the highest bond strength.[17]
Whereas, Sensi et al (2005) have asserted that self etch and total etch primer showed comparable dentin bond strength.[4]
Among the self etching adhesive systems tested, Xeno V (Group II) showed the highest bond strength compared to all other experimental groups i.e. G-Bond (Group IV) and Adper SE Plus (Group III) and the results came out to be statistically significant (p<0.05).
“Strong” self-etch adhesives usually have a pH of 1 or below. This high acidity results in rather deep demineralization effects. At enamel, the resulting acid etch pattern resembles a phosphoric-acid treatment following an etch & rinse approach. At dentin, collagen is exposed and nearly all hydroxyapatite crystals are dissolved (B. Van Meerbeek et al, 2003).[24], [29]
The good bond strength values obtained with Xeno V, which is an intermediately strong self etch adhesive, has been attributed to its acidic pH of 1.4. This acidic nature results in better micromechanical interlocking to enamel and dentin compared to mild self etch adhesives [30]. Low pH ensures etching through thick smear layers to engage underlying intact dentin to form thin zones of demineralization that are simultaneously infiltrated by resinsto provide strong adhesion. It is also suggested that the residual hydroxyapatite at the hybrid layer base may still allow for chemical intermolecular interaction.[29]
The self etching systems which were tested in different studies showed significant differences in performance in terms of bond strength. These differences could be attributed to the efficiency of their respective monomers at infiltrating the smear layers and producing resin tags.[18], [31]
Two-step self-etch system, showed a superior in vitro performance in comparison with one-step self-etch systems [2]. Adper SE Plus (Group III) which is two step self etching adhesive resulted in higher shear bond strength than other self etch adhesive i.e. G-Bond (Group IV), even though it showed low bond strength compared to Xeno V (Group II) (p<0.05). Achievement of strong micromechanical bonding depends on the depth of monomer penetration into demineralized dentin (Erikson, 1992). The superior bonding of Adper SE Plus can be attributed to this two-step procedure for dentin bonding wherein first step prepares and conditions the tooth surface leading to more depth of penetration and in a way mimics total etch technique but with a different mechanism. The second step produces identical depths of etching and penetration of the adhesive. Nanoleakage resulting from an insufficient penetration depth of the adhesive can be minimized by this mechanism. In addition, this keeps the collagen fibers from collapsing and eliminates dependence on “moist bonding” characteristic of the 5th generation systems. The adhesive part of Adper SE Plus Self-Etch Adhesive contains phosphoric acid esters, which under aqueous conditions will etch the surfaces of dentin and enamel to allow for the micromechanical bonding to the tooth. Adper™ SE Plus Self-Etch Adhesive contains a bonded zirconia nanofiller which helps to develop a uniform film, which in turn can lead to higher bond strengths [32].
Single bottle adhesives are complex mixtures of hydrophilic and hydrophobic resin monomers dissolved in water / solvent combinations. Most single step self etch adhesives contain hydroxyethyl methacrylate (HEMA), hydrophilic primer, and the presence of HEMA is advisable for maintaining resins in solution and preventing phase separation[33] that is, it helps to mix hydrophobic and hydrophilic components into one single solution, as well as it serves as a co-solvent to dissolve the diverse active ingredients into water . Self-etch adhesives need water for providing an ionization medium to enable self-etching [33]. On the other hand, HEMA lowers the vapor pressure of water when added to a water mixture, making it more difficult to remove water from the adhesive and retaining water within the adhesive layer[34]. So, HEMA being hydrophilic holds water within the adhesive layer, thereby hindering the polymerization process and thus be detrimental to the bond on the long term. It promotes water to be bonded in unstable soft hydrogels prone to hydrolytic degradation [33]. It even enhances water uptake from the tooth as well as the outer oral environment, rendering the bond more prone to degradation with time [5], [27] Hence, HEMA free single step self etch adhesives were developed (G-Bond).
In accordance with the results of the present study, the lowest shear bond strength was obtained by the self etching HEMA free adhesive G-bond (Group IV). The consequence of the HEMA-free formulation of G-Bondis that upon evaporation of the acetone solvent (once supplied within the cavity), the adhesive monomers separate from the water content (Van Landuyt et al., 2005). Following this phase separation, water droplets are formed within the adhesive layer. The convergence of small blisters into larger ones tends to produce honeycomb structures that may jeopardize the bonded interface [27], [33].This could be the probable reason for the lowest shear bond strength of G-Bond in the present study. Moreover, G-Bond contains 4-methacryloxyethyl trimellitic acid (4-META) which has a weaker bonding potential (Yoshida Y. et al, 2004) [35].
Of all the adhesive system tested, the total-etch Prime & Bond NT provided a highest shear bond strength and among the self etching adhesives Xeno V showed the highest bond strength compared to all other experimental groups.
Within the limitations of this in-vitro study it can be concluded that all the adhesive agents evaluated showed optimal shear bond strength. However, total etch adhesives recorded a higher shear bond strength than the newer self etching bonding agents. But the existing self-etch adhesives are popular because they are easy to handle, convenient, and less confusing for the clinician than the multistep adhesive systems and also as their bond strength lies in the optimal range for clinical success [36].
Even though dental adhesives have been available over several generations with an array of new materials being launched every year, clinical studies are not abundant in the literature. There has been frequent practice among manufacturers to launch a new version of a specific adhesive even before the previous one has been fully tested. As a result of all these limitations, clinicians still rely on data from laboratory studies to predict the behaviour of adhesive materials. Hence, since the materials that were used in the present study were relatively new, further studies need to be done for further clinical evaluation.
Conclusion
Within the limitations of this in vitro study, it can be concluded that all the adhesives agents evaluated showed optimal shear bond strength of 17-20 MPa. However, the one bottle total-etch adhesive Prime & Bond NT recorded higher shear bond strength than the newer self etch bonding agents. In this study, it was seen that among the self etch adhesives, Xeno V showed the highest shear bond strength and G-Bond showed the lowest shear bond strength.
Therefore, this in-vitro study concluded that shear bond strengths of dentin bonding agents with old concept of two-step total-etch technique are superior to that of newer self-etch systems, although their bond strength values also lies within the acceptable range of clinical requirements.
References
1. Luhrs A-K, Guhr S, Schilke R, Borchers L, Geurtsen W, Gunay H (2008) Shear bond strength of self-etch adhesives to enamel with additional phosphoric acid etching. Oper Dent. 33-2: 155-162.
2. Chopra V, Sharma H, Prasad S. Datta (2009) A comparative evaluation of the bonding efficacy of to-step vs all-in-one bonding agents – An in-vitro study. J Conserv Dent 12: 101-104.
3. Naughton W, Latta M (2005) Bond Strength of Composite to Dentin Using Self Etching Adhesive Systems .Quintessence Int. 36: 259-262.
4. Sensi LG, Lopes GC, Monterio S, Baratieri Jr. LN, Vieira LCC (2005) Dentin bond strength of self-etching primers/adhesives. Oper Dent. 30-1: 63-68.
5. Tay F, Pashley D, Suh B, Carvalho R, Itthagarun A (2002) Single step adhesives are Permeable membranes. J Dent. 30: 371-382.
6. Christensen Gordon. J (2001) Self –etching primers are here. J Am Dent Assoc. 132: 1042-1043.
7. Kimmes NS, Barkmeier WW, Erickson RL, Latta MA (2010) Adhesive bond strengths to enamel and dentin using recommended and extended treatment times. Oper Dent. 35(1): 112-119.
8. Meerbeek BV, Inokoshi S, Braem M, et al (1992) Morphological aspect of the resin dentin interdiffusion zone with different dentin adhesive systems. J Dent Res 71: 1530 – 1540.
9. Nakabayashi N, Pashley DH (1998) Hybridization of dental hard tissues Quintessence publishers. Tokyo.
10. Oilo G (1993) Bond strength testing – What does it mean? International dental Journal 43(5): 492-498.
11. Mason P, Ferrari M, Cagidiaco C, Davidson C (1996) Shear bond strength of four dentin bond adhesives applied in vivo and in vitro. J Dent. 24(3): 217-222.
12. Nikaido T, Kunzelmann K.H, Chen H, Ogata M et al (2002) Evaluation of thermal cycling and mechanical loading on bond strength of a self-etching primer system to dentin. Dent Mater. 18(3): 269-275.
13. International Organization for Standardization (1994) ISO TR 11405; Dental materials – guidance on testing of adhesion to tooth structure Geneve: International Organization for Standardization.
14. Senawongse P, Sattabanasuk V, Shinada Y, Otsuki M, Tagami J (2004) Bond strengths of current adhesive systems on intact and ground enamel. J Esthet Restor Dent. 16(2): 107-116.
15. Mithra N Hegde, Shruti Bhandary (2008) An evaluation and comparision of shear bond strength of composite resin to dentin, using newer dentin bonding agents. J Conserv Dent 11(2): 71-75.
16. Retief D, Mandras R, Russell C (1994) Shear bond strength required to prevent microleakage at the dentin-resin interface. Am J Dent 7: 43-46.
17. Kiremitci A, Yalcin F, Gokalp S (2004) Bonding to enamel and dentin using self–etching adhesive systems. Quint Int. 35: 367-370.
18. Bouillaguet S, Gysi P, Wataha J.C, Ciucchi B, Cattani M, Godin C, Meyer J.M (2001) Bond Strength of composite to dentine using conventional, one step, and self etching adhesive systems. J Dent. 29: 55-61.
19. Kerby RE, Knobloch LA, Clelland N, Lilley H, Seghi R (2005) Microtensile Bond Strengths of One-step and Self–etching Adhesive Systems. Oper Dent. 30(2): 195-200.
20. Chuang S, Chang L, Chang C, Yaman P, Liu J (2006) Influence of enamel wetness on Composite restorations using various dentin bonding agents; Part 2 – effects on shear bond strength. J Dent. 34:352-361.
21. Ogata M, Okuda M, Nakajima M, Pereira P, Sano H, Tagami J (2001) Influence of the direction of tubules on bond strength to dentin. Oper Dent. 26: 27-35.
22. Turgut M.D, Tekcicek M, Olmez S (2004) Clinical evaluation of a polyacid-modified resin composited under different conditioning methods in primary teeth. Oper Dent. 29(5): 515-523.
23. Ritter A.V, Heymann H.O, Swift Jr E.J, Perdigao J, Rosa B.T (2000) Effects of different re-wetting techniques on dentin shear bond strengths. J. Esthet Dent. 12: 85-96.
24. Norbert Moszner, Ulrich salz, Jorg zimmermann (2005) Chemical aspects of self- etching enamel-dentin adhesives: A systematic review. Dental materials 21: 895-910.
25. Tate W.H, You C, Powers J.M (2000) Bond strength of compomers to human enamel. Oper Dent. 25: 283-291.
26. Riekurokawoa, Werner J, Finger B, Marcus Hoffmann C (2007) Interactions of self-etch adhesives with resin composites. J Dent. 35: 923-929.
27. Salz U, Zimmermann J, Zeuner F, Moszner N (2005) Hydrolytic stability of self-etching adhesive systems. J Adhes Dent. 7: 107-16.
28. Pashley DH, Carvalho RM (1997) Dentin permeability and dentin adhesion. J Dent. 25: 355-372.
29. Meeerbeek Van B, De Munck J, Yoshida Y, Inoue S. Vargas M, Vijay P, Van K. Landuyt, Lambrechts P, Vanherle G (2003) Buonocore Memorial Lecture Adhesion to Enamel and Dentin: Current Status and Future Challenges. Oper Dent. 28(3): 215-235.
30. Meerbeek B V, Conn LJ. Jr, Duke ES, Eick JD, Robinson SJ, Gueerero D (1996) Correlative transmission electron microscopy examination of non demineralized and demineralized resin-dentin inferfaces formed by two dentin adhesive system. J Dent Res. 75: 879 – 888.
31. Prukkanon S, Burrow MF, Tyas MJ (1999) The effect of dentine location and tubule orientation on the bond strengths between resin and dentine. J Dent. 27: 265-74.
32. 3M ESPE Adper SE Plus Self-etch Adhesive. Product Guide.
33. Monticelli F, Osorio R, Proemca J, Toledano N (2007) Resistance to degradation of resin-dentin bonds using a one step HEMA – free adhesive. J Dent. 35: 181-186.
34. Hiraishi N, Breschi L, Prati C, Ferrai M, Tagami J, King N (2007) Technique sensitivity Associated with air drying of HEMA-Free, single bottle, once step self etch adhesive. Dent Mater. 23: 498-505.
35. Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, Inoue S, Tagawa Y, Suzuki K, Munck J. De and Meerbeek B.V (2004) Comparative Study on Adhesive Performance of Functional Monomers. J Den Res.83(6): 454- 458.
36. Lopes G, Baratieri L, Andrada C, Vieira C (2002) Dental adhesion: present state of the art and future perspectives. Quintessence International33:213-224.
|