Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common malignancy and a major cause of cancer morbidity and mortality globally (1). High incidence of these cancers is seen in the Indian subcontinent, Australia, few European countries (Netherlands, France, Switzerland), Brazil and South Africa (2). Studies show male preponderance in France but a female predominance in India and Pakistan (3). Cancers of the mouth and the tongue predominate in developing countries whereas pharyngeal cancers are common in developed countries including Central and Eastern Europe. The mainstay of management remains surgery, radiation and chemotherapy. However, despite advances in screening tools, imaging technology and access to primary care physicians, a considerable percentage of patients present with advanced stage disease. Survival of oral cancer has not improved in the past 30 years and hence there is still a substantial gap in the management of oral cancer and a need for an effective vaccine to prevent oral cancer cannot be overlooked. Recent developments in the field of immunology have resulted in a vaccine to treat patients with HPV against cervical cancer. HPV is accepted as an etiological factor for oral and pharyngeal cancers. Numerous trials have studied the prevalence of HPV in OSCC and a largest study on this aspect calculated the average to be 4% for cancers of the oral cavity and 18% for oro-pharyngeal cancers (4). Kreimer et al (5) showed HPV-16 was the predominant type in 87% and 68% cases of HPV infected oro-pharyngeal and oral cancers respectively. Hence a possible reduction in OSCC as a result of widespread vaccination against HPV appears promising, as a good proportion of the infection is caused by HPV types and therefore potentially preventable.
Tumor vaccine research
HPV is non-enveloped with a 8.0 kb circular genome that encodes two structural proteins (L1 and L2) that form the viral capsid and more than 100 strains have been identified (6). The virus gains entry through a micro trauma to the superficial epithelial layer and goes on to invade the basal epithelial layer. It has the ability to evade the immune system and thereby limiting gene expression and viral replication to the suprabasilar layers (7). The HPV virus appears to have a latency period of 10 years or more before malignant transformation occurs. The discovery, development and testing of the two highly promising HPV vaccines (Table 1)
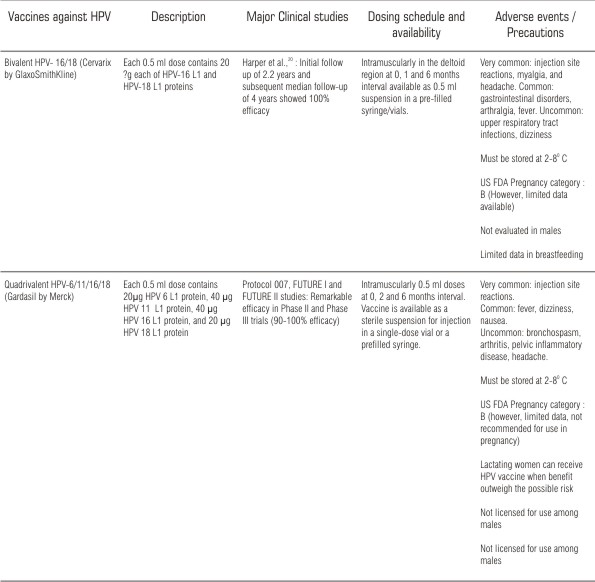 | Table 1. Currently available HPV vaccines
 |
is a major breakthrough in modern preventive medicine (8-10). HPV vaccine is a non-infectious, subunit, viral vaccine based on the L1 major capsid protein. When expressed in eukaryotic cells, L1 proteins are able to self-assemble into virus-like particles (VLP) that are very similar to authentic virions and can induce high titers of antibodies able to prevent infection by native virions (9). VLPs can be produced in both yeast cells and baculovirus-infected insect cells. The VLP based vaccine is type-specific and can therefore target the HPV genotype most commonly associated with the development of oral cancer (7). HPV types 16 and 18 are responsible for majority of oral cancers and so, monovalent and/or polyvalent vaccines, which target one or more of these types would provide the most beneficial and efficient protection against HPV (11). Both vaccines are administered as an intramuscular series of three injections over a six-month period.
Efficacy of tumor vaccine against oral cancer
Available HPV vaccines, at present are prophylactic and not therapeutic. Numerous studies done till date discuss the effect of HPV vaccines against cervical cancer alone and do not address the issue related to tumour vaccine specifically for oral cancer. It is surprising indeed, that though OSCC is a common cancer there are only a handful of studies dealing on this issue. One of the recent studies investigated immunomodulatory activity of autologous tumour cell vaccine from oral cancer patients ex vivo by lymphoproliferation assay and two color flow cytometry. Vaccine treatment lead to 10-fold higher proliferation of lymphocytes compared to untreated controls (12). This finding of lymphocyte proliferation is definitely a positive prognostic factor for oral cancer patients. Another study 'throws up' a promise in the prevention of HPV associated oral cancer with the help of L1 DNA vaccines (13). Due to lack of further studies pertaining to vaccines in oral cancer, the exact impact of HPV in prevention of oral and oro-pharyngeal cancers and other benign lesions is not known. As the overall 5-year survival rate for OSCC is only approximately 55%, if a vaccine could confer long-term immunity and reduce the incidence of oral cancer, it will have a huge impact on the society. Also, HPV is considered to be a sexually transmitted due to abnormal sexual practices. Sex education, improvement in personal hygiene and vaccination in high risk groups can also add on to bring down the incidence of oral cancer (14). Dentists can play a crucial role through effective counseling pertaining to safe sexual practice and oral hygiene.
Gaps in knowledge and research
There is a substantial gap in knowledge and research when tumour vaccines move from bench side to bed side. Though an optimal age group for vaccination is not clearly defined, immunological bridging studies have documented better serological responses to the quadrivalent vaccines among 9-15 year old females than among the older group (15). US FDA has recently approved the quadrivalent vaccine for use in women between 9-26 years of age (6). Till to date, there is no data focusing the efficacy of HPV vaccine in males. However, the burden of HPV-16 associated penile, anal and oropharyngeal cancers in men are not significant and considerably less than the HPV 16/18 associated cervical disease in women (16). Though HPV vaccine generates VLPs that induce strong immune response that is protective against persistent HPV infection, the durability of response imparted by HPV vaccine and the timing of a booster dose is not clear and can be determined only by monitoring antibody levels to HPV infections in immunized subjects (17).
Some of the overlooked areas in research include
(a) the extent of cross-type neutralization induced by VLPs in in-vitro assays
(b) immune protection, status of immune response post-vaccination
(c) to determine if herd immunity is induced
(d) the exact duration of protection, degree and duration of cross protection against the types not included in the vaccine
(e) determination of safety and efficacy of the vaccines in elderly, HIV positive and other immunocompromised individuals
(f) determining the impact of vaccination on prevalent infections especially the anogenital and oral infections.
Figure 1 summarizes the research gaps and challenges ahead in vaccine research against oral cancer.
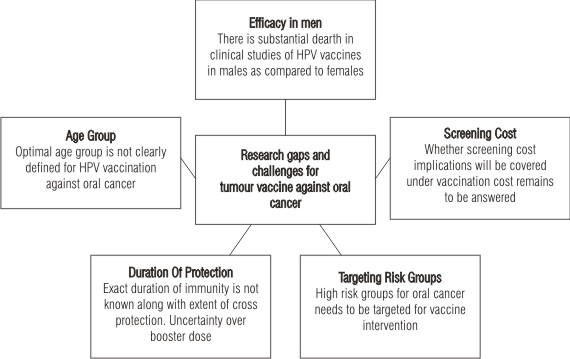 | Figure 1: Tumor vaccine against oral cancer: the challenges
 |
Clinical evaluation and endpoints for tumor vaccines
It is important to know the endpoints for judging the therapeutic efficacy of HPV vaccines against oral cancer. Vital perquisites for all vaccines as endpoints are (a) immunogenicity (B and T cell responses), (b) reactogenicity (local and systemic reactions), (c) safety (short and long term adverse events) and (d) protection (clinical trials efficacy and effectiveness in population use). Clinical endpoints can be assessed through physical examination, by cytology through Papanicolaou testing or histologically through biopsy sampling. Endpoints for HPV vaccine against cervical cancer are well defined, but for oral cancer it remains under review. All adverse events occurring during the clinical trials with HPV vaccines are registered and their frequency is determined. Causality between the adverse events and vaccination are evaluated (18). Psychosocial impacts are often neglected as a part of screening the patients with oral cancer. Decrease in anxiety may be a positive benefit of vaccine administration for which definite endpoint need to be defined (19, 20). National immunization advisory committees in various countries can play a crucial role while facilitating HPV vaccine administration against oral cancer.
Conclusion
Although there is considerable lack of tumour vaccine studies focusing on oral cancer, the substantial benefit of preventing oral cancers through vaccine administration cannot be overlooked. Need for research in this area with focus on efficacy, safety and post-marketing surveillance is crucial. Multidisciplinary approach with dentists in particular, needs to be sensitized with the latest advancement in tumour vaccine research. A subtle shift from bench side research to bed side availability of an effective tumour vaccine in the armamentarium of dental professionals does not seem to be a distant dream.
References
1. Das BR, Nagpal KJ. Understanding the biology of oral cancer. Med Sci Monit 2002;8:RA258-67.
2. Parkin DM, Bray F. Chapter 2: The burden of HPV related cancers. Vaccine 2006;24 Suppl 3:S11-25.
3. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents. Vol 8. Lyon: IARC Scientific Publications, 2002.
4. Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-83.
5. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467-75.
6. Sussman AL, Helitzer D, Sanders M, Urquieta B, Salvador M, Ndiaye K. HPV and cervical cancer prevention counseling with younger adolescents: implications for primary care. Ann Fam Med 2007;5:298-304.
7. Lea JS, Sunaga N, Sato M, Kalahasti G, Miller DS, Minna JD, et al. Silencing of HPV 18 oncoproteins with RNA interference causes growth inhibition of cervical cancer cells. Reprod Sci 2007;14:20-8.
8. Wright TC, Bosch FX, Franco EL, Cuzick J, Schiller JT, Garnett GP, et al. Chapter 30: HPV vaccines and screening in the prevention of cervical cancer: conclusions from a 2006 workshop of international experts. Vaccine 2006;24 Suppl 3:S251-261.
9. MICROMEDEX ® Healthcare Series, Thompson Micromedex, Greenwood Villange, Colorado. Vol.133 expires 9/2010.
10. Quadrivalent Human Papillomavirus Vaccine. Recommendations of the advisory committee on immunization practices - March 12, 2007. 'http://www.cdc.gov/mmwr/preview/mmwrhtml/rr56e312a1.htm'. Accessed April 2010.
11. Marais DJ, Sampson C, Jeftha A, Dhaya D, Passmore JA, Denny L, et al. More men than women make mucosal IgA antibodies to Human papillomavirus type 16 (HPV-16) and HPV-18: a study of oral HPV and oral HPV antibodies in a normal healthy population. BMC Infect Dis 2006;6:95.
12. Agarwal A, Mohanti BK, Das SN. Ex vivo triggering of T-cell-mediated immune responses by autologous tumor cell vaccine in oral cancer patients. Immunopharmacol Immunotoxicol 2007;29:95-104.
13. Maeda H, Kubo K, Sugita Y, Miyamoto Y, Komatsu S, Takeuchi S, et al. DNA vaccine against hamster oral papillomavirus-associated oral cancer. J Int Med Res 2005;33:647-53.
14. Palefsky JM. HPV infection in men. Dis Markers 2007;23:261-72.
15. Lajiness MJ. The new vaccine to prevent HPV. Urol Nurs 2007;27:153-4.
16. Zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst 2000;92:690-8.
17. Franco EL, Bosch XF, Cuzick J, et al. Chapter 29: Knowledge, gaps and priorities for research on prevention of HPV infection and cervical cancer. Vaccine 2006;24 Suppl 3:S242-9.
18. Wood D, Shin J, Duval B, Schmitt HJ. Chapter 22: Assuring the quality, safety, efficacy of HPV vaccines: The scientific basis of regulatory expectations pre- and post-licensure. Vaccine 2006;24 Suppl 3:S187-92.
19. Shinn DL. Fear and anxiety. Br Dent J 2007;202:2.
20. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006; 367:1247-55.
|