Introduction
Oral Squamous Cell Carcinoma (OSCC) is a serious health hazard that affects people of all sectors of the society. It ranks the 6th most common neoplasm in the world today & accounts for about nine of every ten oral malignancies.[1] The prognosis of the patients affected by this disease remains relatively poor owing to the differential biologic behaviour & variable aggressiveness exhibited by the neoplasm. The 5year survival rate being less than 59%. [2]
Recent studies suggest that tumor that shows lympho-vascular permeation (nodal extracapsular invasion), perineural invasion and muscular invasion is associated with poor prognosis. These tumors have a high tendency to develop second primary tumors and are associated with high loco-regional recurrence.[3] Therefore, detecting the mechanisms that would help in cancer cell invasion and metastasis is a need of the hour.
Research in the field of oncology suggests that, various growth factors produced by the tumor cells play a role in the process of angiogenesis and invasion, one of the growth factor that helps in both these functions is the fibroblast growth factor.
Fibroblast growth factors, or FGFs, was first found in pituitary extracts by Armelin in 1973 and was also found in a cow brain extract by Gospodarowicz et al. When tested in a bioassay, it caused the proliferation of fibroblasts. Later it was fractionated in acidic and basic pH and two isoforms of the growth factor was obtained which were called acidic and basic fibroblast growth factor.
Also, known as "pluripotent" growth factors, FGF have regulatory, morphological, and endocrine function. They are the key players in the process of proliferation and differentiation of wide variety of cells and tissues like mesodermal and neuroectodermal cells.Studies have shown that bFGF promotes the production of cancer cell proteinases & increases their invasive ability.[4] Culture cell study suggest that bFGF increases collagenases and plasminogen activators that are important for angiogenesis and tumor invasion.Its overexpression plays a key role in the progression of OSCC.[5]
Thus, bFGFs is hypothesized to play a role in OSCC formation and progressionbut their exact function is unclear. An attempt is made to study the role of bFGF on invasive capacity of OSCC and correlate its expression in different histologic grades of OSCC & with lymphnode metastasis.
Materials & Methods
The Institutional Ethics Committee approved the study.
The study sample included 34 formalin fixed paraffin embedded tissue blocks retrieved from the archives of the Department of Oral Pathology, Government Dental College, Calicut. Nodal status of all the cases was obtained from the archival records. 5 cases of normal buccal mucosa specimens of healthy individuals undergoing impacted tooth removal were taken as control with informed consent. Of the 34 blocks, 10 cases were histologically diagnosed as well differentiated squamous cell carcinoma (WDSCC), 12 cases were moderately differentiated (MDSCC) and 12 cases were poorly differentiated squamous cell carcinoma (PDSCC). Cases without tumor invasive front were excluded from the study. These cases were classified according to Yammomoto’s classification to assess the invasion pattern of the carcinoma.(Annexure-I). 5 µm thick sections were cut from all the blocks for immunostaining with bFGF
Immunohistochemistry
Briefly, sections were dewaxed with xylene and rinsed in a series of graded alcohols. Endogeneous peroxidase activity was blocked with 3% hydrogen peroxidase for 20 minutes. Antigen retrieval was done using microwave. Afterwards, sections were incubated with mouse anti bFGF monoclonal antibody, (Biogenex) pH-7.6.An appropriate volume of super enhancer reagent was added to cover the specimen and incubated for 20 minutes at room temperature. The sections were rinsed well with TBS and were dried by blotting. They were then incubated with biotinylated secondary antibody for 30 minutes at room temperature followed by rinse with 3 changes of TBS, drained and blotted gently around the sections. Sections were covered with working solution and incubated for 15 minutes at room temperature. Washed with distilled water for 5 minutes. Slides were counterstained with Mayer’s haematoxylin for 1 minute. Washed in running tap water for 5 minutes and slides were dehydrated, cleared and mounted with DPX.
Scoring and Statistical Analysis:
For evaluation of bFGF expression in squamous cells, the slides were examined under a compound microscope at 400x magnification by two observers simultaneously using a biheaded microscope. Three high power fields were selected randomly in each section and the intensity of bFGF expression in squamous cells at the tumor front was evaluated by comparing it with basal layer of epithelium/ skeletal muscle (internal positive control). Normal buccal mucosa sections stained with bFGF was used as an external positive control. A negative control was performed in all cases by subtracting the primary antibody. Brown staining of the cytoplasm of cancer cells was considered positive. If cancer cell staining for bFGF is same as that of the control then it was coded as 2; if less than the control then it was scored as 1; if more than control then scored as 3. The mean value for each case was calculated.
bFGF expression in fibroblasts at the tumor invasive front was also calculated. Positive expression was considered if 25% of cells at the invasive front partially or completely expressed bFGF. This expression was compared between different grades of Oral squamous carcinoma.
bFGF expression in tumor cells and fibroblasts was independently compared and correlated in different histologic grades of OSCC, different grades of invasion and lymph nodes. Statistical analysis was performed using Spearman’s correlation test and p-value less than 0.05 was considered significant. For statistical analysis, SPSS software was used.
Results
bFGF expression was cytoplasmic in most of the cells and occasionally showed nuclear staining in few cancer cells. In 29.4% (n=10) of WDSCC, intensity of bFGF expression was 1.1167 ± 0.13 SD, in 35.3% (n=12) of MDSCC, bFGF expression was 1.83±0.27 and in 35.3% (n=12) of PDSCC bFGF expression was 2.55±0.3. (Table-1)
When the same cases were graded according to Yammomoto’s classification, 17.6% (n=6)cases were classified as grade I and showed a mean bFGF intensity of 1.05±0.86, 20.6%(n=7) of grade II showed 1.54±0.3, 29.4% (n=10)cases of grade III showed 1.9±0.52, 14.7%(n=5) of grade IVC exhibited 2.4±0.3, 17.6%(n=6) cases of grade IVD showed 2.61±0.33. (Table-2)
On comparing the lymph node status with bFGF, 79.4%(n=27) of cases with nodal involvement showed bFGF intensity of 1.93, and in non nodal involvement cases 20.6%(n=7) bFGF expression was 1.43. (Table-3)
Mean bFGF intensity in fibroblasts was 0.15±0.28 in 29.4% of WDSCC, and 0.55±0.4 in 35.3% of cases, 0.56±0.3 in 35.3% of PDSCC. (Table-4)
Positive staining was also noticed in endothelial cells, few fibroblasts, lymphocytes. When present, salivary glands occasionally took up stain.
 | Anexure 1- Histologic grading of mode of cancer invasion(Yamamoto et al)
 |
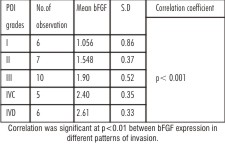 | Table 2-Pattern of invasion (POI) in comparison with bFGF expression.
 |
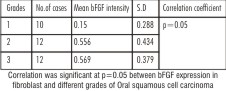 | Table 4 - Mean and Standard deviation of Fibroblasts with respect to Grades
 |
Discussion
FGF family of growth factors comprises of 22 members, all of which are structurally related signaling molecules and bind to fibroblast growth factor receptors (FGFRs) namely FGFR1, FGFR2, FGFR3, and FGFR4.
Fibroblast growth factors play important role in various physiological conditions like wound healing, where it stimulates the proliferation of fibroblasts & gives rise to granulation tissue that fills up the wound space early in the wound healing process. It also stimulates mRNA, DNA, and protein synthesis in fibroblasts and epithelial cells. It helps in angiogenesis by promoting the proliferation & organization of endothelial cells into tube-like structures. This ability of FGF’s to induce angiogenesis also helps in development & progression of pathological conditions like cancer. Of all the fibroblast growth factors, FGF2, also known as basic fibroblast growth factor (bFGF), has angiogenic properties and is highly chemotactic and mitogenic for a variety of cell types.
Researchers in the field of oncology, have found that basic fibroblast growth factor, plays a significant role in the development of many tumors like astrocytoma, and breast carcinoma.[6],[7],[8] Since, its role in oral squamous cell carcinoma is largely unknown, the study was conducted to correlate the expression of bFGF with varying grades of Squamous cell carcinoma, and invasion status, and lymph node involvement.
Under normal conditions we observed that, non-keratinising squamous epithelium showed uniform cytoplasmic expression of bFGF protein in the superficial layers with more intense labelling in the basal layers. This is in accordance with the earlier literature. Recent studieshave suggested that the receptors of bFGF namely FGFR1 and FGFR2 is located in the more superficial layers and indicated that bFGF is involved in the differentiation of squamous cells.[9] We also noted the expression of bFGF in the nucleus of few cells. This nuclear expression could be due to the presence of high molecular weight isoforms of bFGF or due to the presence of FGFR3 present in the nucleus.[9]
In our study it was shown that the intensity of bFGF expression in tumor cells of OSCC was significantly higher than in normal epithelium and when correlated with different grades, it showed greater expression in higher grades which is in agreement with the previous study.[6]
We are of the opinion that as tumor cells in MDSCC and PDSCC have lost their regulatory control, they express a high levels of bFGF. But in the WDSCC, tumor cells are terminally differentiated, and have functional similarity with normal epithelium; they express bFGF in low amounts compared to higher grades. Most research conducted on lung cancer, oesophageal malignancy, idiopathic pulmonary fibrosis indicated that high expression of bFGF is related to poor outcomes, increased risk of recurrence and reduced survival.[6],[10]
Comparison of bFGF expression in tumor cells in different grades of invasion (Yamommato’s classification) at the invasive front, yielded that bFGF expression was less in low grade carcinomas (grade I & II consisting of islands of cells) and was high in grade III, IVC and IVD, which comprised of diffuse type of invasion and isolated cells and strands. The cells in the grade IVC and IVD were poorly differentiated and had a highly mutated genotype, they exhibited a high expression of this protein. This observation was similar to the previous reported experiments.[11]
However, Forootan et al in their observations had significant correlation of bFGF with invasion status but no correlation was seen with different grades of OSCC. They concluded that bFGF promoted the growth and development of primary carcinomas and their metastases.[9] In another study, Ali.et al also showed that no significant difference existed between expression of angiogenic factors like bFGF, VEGF, PDGF and histologic grades of OSCC. They have not explained this difference in expression but had suggested that bFGF was important in the growth of the carcinoma and its supporting stroma.[12]
Experiments conducted on bFGF, demonstrated that, it promotes the production of cancer cell proteinases, collagenases and in association with heparanase causes the breakdown of heparin sulphate proteoglycans, a component of extracellular matrix protein and helped in cancer cell invasion and metatsasis.[1],[5] Invitro studies conducted on human squamous carcinoma cell lines noted a rapid endothelial cell turnover rate in bFGF positive areas, suggesting that a correlation exists between the proliferative capacity of tumor and bFGF expression.[13] In addition, it increases cancer cell survival by inhibiting apoptosis via survivin (an inhibitor of apoptosis) and also inhibits many other proapoptotic proteins.[14] Thus elevated levels of bFGF produced by cancer cells in higher grades helps in mitotic division of the cells and break the extracellular matrix and helps in cancer cell invasion and metastasis.
Szhult Hector reported that bFGF expression in fibroblasts at the tumor invasive front correlated with grades of OSCC and invasion status. They believed that bFGF produced by tumor cells not only acted in autocrine manner on themselves and helped in their invasion but also acted in a paracrine fashion on fibroblast and helped in further breakdown of extracellular matrix by releasing collagenases and helped in further invasion and metastasis.[13] In our study also, an increased levels of bFGF expression in fibroblasts with increasing grades of OSCC was demonstrated and correlated with the invasion status of the tumor. In earlier studies, the authors had studied the expression of FGF receptors in fibroblast and could establish a correlation between bFGF expression in tumor cells and receptors present on fibroblasts.[11] In the present study since expression of FGFr has not been studied, we were not able to establish a correlation between tumor cell bFGF & fibroblast bFGF.
Lymph node involvement is an important prognostic factor of carcinomas. In our study, bFGF expression was correlated with the lymph node involvement and although 70% of our cases were positive for bFGF and lymph node status, the result was not statistically significant. This lack of correlation between bFGF and lymph node involvement observed in our study is contrary to the findings of Forootan et al.[9] The limited sample size of our study combined with lack of adequate histological records regarding the nodal status of the cases could be the reason for this contradictory finding.
The results of our study implicate that there is an upregulation of bFGF with increasing histologic grades of differentiation indicating an increase in the invasive capacity of cancer cells. It also helps in cancer cell survival by increasing vascularity.
Conclusion
Our results support the hypothesis that bFGF is expressed in OSCC and it increases with increasing grades of differentiation. Over expression of this protein is associated with higher invasiveness of OSCC. Increased levels of this growth factor could enhance tumor cell survival by inducing angiogenesis and can help in tumor growth and invasion by destruction of extracellular matrix. Thus inhibition of bFGF by antiangiogenic factors may be a way for cancer control. We suggest that if future studies are conducted to evaluate the relationship between bFGF expressed by tumor and fibroblast bFGF using receptor markers, a better insight into the understanding of importance of tumor stroma in carcinogenesis can be obtained.
References
1. Chen Zhong, Zheng Xiaodong, Feng Hongchao. The expressions and significance of Hpa and bFGF in oral squamous-cell carcinoma. Chinese-German Journal of Clinical Oncology. 2009; 8: 46–49
2. Sudbo Jon, Kildal Wanja, Risberg Bjorn. DNA content as a prognostic marker in patients with oral leukoplakia. N Engl J Med. 2006;355:1270-8
3. Massano Joao, Frederico. Oral Squamous cell carcinoma: Review of prognostic and predictive factors.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102: 62-76.
4. Hase Takashi, Kawashiri Shuichi Tanaka Akira. Fibroblast growth factor-2 accelerates invasion of oral squamous cell carcinoma. Oral science international 2006;3:1-9
5. D’Armiento J, SiColandrea. Collagenase expression in transgenic mouse skin causes hyperkeratosis , acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol 1995;15:5732-9
6. Jang fateh Wei Wang, De Mao Wang. Vascular endothelial growth factor and basic Fibroblast growth factor expression positively correlates with angiogenesis and peritumoural brain oedema in Astrocytoma J Ayub Med Coll Abbottabad. 2008;20:24-27
7. Reif Michele, Lejeune Susan, Scott A. E Prudence. expression of the Angiogemc Factors Vascular Endothelial Cell Growth Factor(VEGF), Acidic (aFGF) and Basic Fibroblast Growth Factor (bFGF), Tumor Growth Factor beta-1(TGFbeta1), Platelet-derived Endothelial Cell Growth Factor(PDECGF), Placenta Growth Factor, and Pleiotrophin in Human Primary Breast Cancer and Its Relation to Angiogenesis' Cancer Research. 1997;57: 963-969.
8. Dirix L. Y, Vermeulen P. B. Huberts.G. Serum basic fibroblast growth factor and vascular endothelial growth factor and tumour growth kinetics in advanced colorectal cancer. Annals of Oncology 1996;7: 843-48.
9. ForootanS.S, Ke.Y.JonesA.S, Helliwell. Basic fibroblast growth factor and angiogenesis in squamous carcinoma of the tongue Oral Oncology 2000;36: 437-43
10. Barclay Christie, W. Li Audrey. Basic Fibroblast Growth Factor (FGF-2) Overexpression Is a Risk Factor for Esophageal Cancer Recurrence and Reduced Survival, which Is Ameliorated by Coexpression of the FGF-2. Antisense Gene Clinical Cancer Res 2005;11:7683
11. Hase Takashi Kawashiri Shuichi. Correlation of basic fibroblast growth factor expression with the invasion and the prognosis of oral squamous cell carcinoma. J Oral Pathol Med 2006; 35: 136–9
12. Ali A Mohammad. Lymphatic Microvessel Density and the expression of Lymphangiogenic Factors in Oral Squamous Cell Carcinoma Med Princ Pract. 2008;17:486–92.
13. Hector S. Schultz-1 and Haghayegh. S. ß FibroblastGrowth Factor expression in Human and Murine Squamous Cell Carcinomas and Its Relationship to Regional Endothelial Cell Proliferation Cancer research. 1993;53: 14444-49
|