Introduction
Diabetes mellitus is a clinically and genetically heterogeneous group of metabolic disorders manifested by abnormally high level of glucose in the blood. Diabetes Mellitus is a syndrome and it is now recognized that chronic hyperglycemia leads to long term damage to different organs including the heart, retina, nephrons, nerves and vascular system.[1]
The impact of diabetes mellitus on the oral cavity has been well researched. A large body of evidence demonstrated that diabetes is a risk factor for gingivitis and periodontitis.[2],[3] Also poorly controlled diabetes leads to higher prevalence and severity of gingival inflammation and periodontal distruction.[4], [5], [6], [7] Studies have shown that diabetes also increases the risk of alveolar bone loss and attachment loss approximately 3-folds, when compared to non-diabetic subjects.[8],[9] Especially in uncontrolled type 2 diabetes subjects had an 11 fold increase in the risk of alveolar bone loss over a period of 2 years compared to non-diabetic subjects.[10] On the other hand well controlled type 2 diabetes patients had no significant increase in risk of longitudinal alveolar bone loss compared to non-diabetic.
Influence of periodontal disease on diabetes is well known now. As periodontal disease is inflammatory in nature, alter the glycemic control by increasing the cytokines (IL-1β, IL-6, TNF-α) leading to increased glucose production in the hepatocytes and increased insulin resistance leading to hyperglycemia. Diabetic patients with periodontal disease have greater risk of worsening glycemic control over time compared to diabetic subjects without periodontitis,[11] hence control of glucose level is very important as it is the chief cause for all diabetes complications. Glucose levels were controlled by diet, exercise, and pharmacological management. Pharmacological management includes insulin or other oral hypoglycemics like biguinides, sulfonylureas, meglitinides, thiazolidinediones, α-glucosidase inhibitors etc.[1]
Control of infection plays an important role in the glycemic control of diabetes mellitus. As the periodontal disease has been proved to cause a worsening of glycemic control, control of periodontal infection becomes an important aspect of diabetes control.[12] Several studies have addressed the effect of periodontal treatment on metabolic control of diabetes mellitus patients[13], [14], [15] Christgaue and others[13],[16] reported that mechanical therapy had no effect on the level of glycated hemoglobin, similarly in a 5 year maintenance study Westfield et al.[14] found no alterations in HbA1c level in group of controlled and moderately controlled diabetes patients. On the other hand Stewart et al.[15] suggested in a retrospective study that there was a marked improvement in the glycemic control in subjects with type-2 diabetes mellitus following periodontal therapy. Also Faria et al.[17] applied conventional therapy to subjects with and without type-2 diabetes mellitus and found an improvement in the clinical parameters and glycated hemoglobin levels.In diabetes patients with periodontitis, periodontal therapy may have beneficial effect on glycemic control.[8],[19] This may be especially true for patients with relatively poor glycemic control and more advanced periodontal destructions before treatment.[20]
Since early 1980s, tetracycline has been known to inhibit vast number of periodontal pathogens. Herrera D et al.[21] in his review has shown that systemic antimicrobials in conjunction with scaling and root planing, can offer an additional benefit over scaling and root planing alone in treatment of periodontitis, in terms of clinical attachment level and probing pocket depth change, and reduced risk of additional clinical attachment loss.[21],[22] Also a study done by Yoshihiro Iwamoto et al.[23] on the effect of antimicrobial periodontal treatment on circulating Tumor Necrosis Factor alpha (TNF-α) and glycated hemoglobin level in patient with type 2 diabetes on 13 patient concluded that anti-infectious treatment with minocycline is effective in improving metabolic control in diabetes, possibly through reduced serum TNF-α and improved insulin resistance.[19]
So, the studies do not confirm that the periodontal therapy has a definitive impact on the glycemic control of uncontrolled diabetic patients. The aim of the present study is to evaluate the short term effect of periodontal therapy with adjunctive systemic antimicrobials on the glycemic control and periodontal health of uncontrolled type 2 Diabetic patients.
Materials and methods
20 patients with at least 1 site with probing depth ≥ 5mm and 2 teeth with attachment loss of ≥ 6mm (diagnosed as chronic periodontitis), visiting department of periodontology were included in the study. Both males and females were included in study. Patients with chronic generalized periodontitis and type 2 diabetes mellitus with fasting blood sugar level ≥ 126 mg/dl and HbA1c ≥ 7% were included in the study. Exclusion criteria included non diabetic patients, patients under any antimicrobial medications during previous 6 months, pregnant and lactating females, patients taking drugs which would interfere with the periodontium or the response to periodontal therapy, patient who had undergone periodontal therapy within 6 months and patients with history of smoking.
For every patient case history was recorded, clinical examination was done and Silness And Loe Plaque Index(Pi), Loe And Silness Gingival Index (Gi), Probing depth(PD), Clinical Attachment level(CAL), Fasting Blood Sugar level(FBS) and Glycosilated hemoglobin(HbA1c) was recorded.
All the patients were subjected to scaling and root planing and randomly assigned into test group and control group of 10 patients each. Test group received doxycycline 100 mg once daily for 2 weeks, whereas control group received scaling and root planning alone. All the parameters i.e. periodontal parameters (PI, GI, PD, CAL) and blood parameters (FBS, HbA1c) were re-evaluated after 12 weeks. 2 ml of blood was drawn in air tight syringe from anticubital vein of left forearm under aseptic precautions. Fasting blood sugar levels were determined by commercially available reagents and instruments according to GOD-POD, End Point Assay and Kinetic Assay. HbA1c were determined by commercially available reagents through Micro Column method. It is an accurate, reliable method for estimation of average glucose level of previous 3 months.
Statistical Analysis:
All the raw data compiled in Microsoft excel and transferred to the spss software version 17 for statistical analysis. Descriptive data employed to sudy the characteristics of study population. Unpaired t test/ Man’Whitney U test used to compare the various parameters at baseline and 12 weeks between the two groups. Pair t test / Wilcoxon t test analysis was used to assess the change in the parameters from baseline to 12 weeks within each group. Statistical significance is fixed at 5%.
Results:
In the present study 20 subjects participated with the mean age of 43years. We found no significant difference in all the parameters between the groups at baseline. (Table no.1)
When PI and GI were compared from baseline to 3 months, both control and test group showed significant improvement in the scores. Gingival index showed more improvement in the test group than control group, but was not statistically significant. When PD, CAL, FBS and HbA1c were compared from baseline to 3 months both control and test groups showed statistically significant improvement.(Table no.2)
However there was statistically significant reduction at 12 weeks in probing depth, clinical attachment level, fasting blood sugar level and glycated hemoglobin in the test group as compared to control group.(Table no.1)
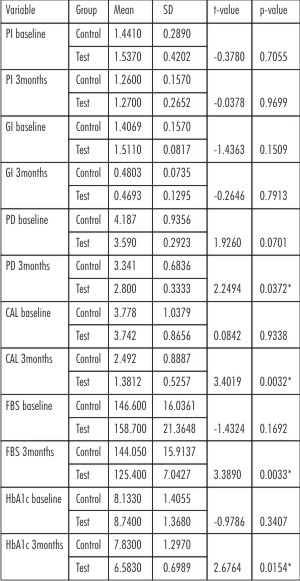 | Table 1: Comparison Of Parameters Between The Groups At Baseline And 12 Weeks
 |
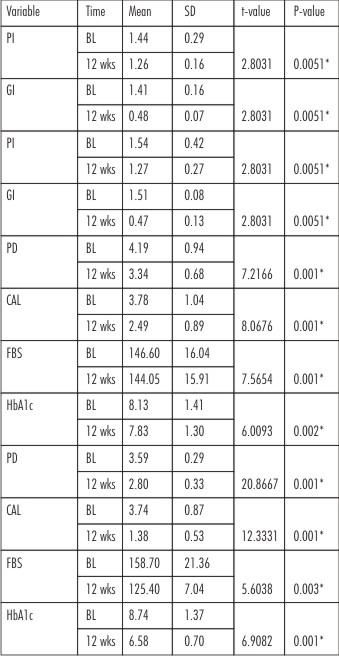 | Table 2: Comparison Of Parameters Within The Groups
 |
Discussion
Diabetes mellitus is a highly prevalent metabolic disorder; with 150 million cases estimated worldwide, it constitutes a global public health burden.[25],[26],[27] Diabetes is divided into two main forms: type 1 diabetes mellitus (formerly insulin-dependent diabetes mellitus) and type 2 diabetes mellitus (formerly non-insulin-dependent diabetes mellitus). Type 1 diabetes is caused by immune-mediated destruction of the insulin-producing pancreatic β cells and accounts for 10% to 15% of all diabetes cases. The more common form, type 2 diabetes, results from a combination of impaired insulin production and insulin resistance. Both forms of the disease are associated with a range of complications that increases the morbidity and mortality of affected individuals.[27],[28] These complications ensue from abnormal regulation of glucose metabolism that characterizes diabetes.
In the macrovasculature, accelerated and aggressive atherosclerosis portends the development of premature cardiovascular and cerebrovascular events. Microvascular disease may lead to the devastating complications of blindness and renal failure. In certain complications, such as impaired wound healing, impotence and neuropathy, dysfunction of vascular, inflammatory and neural components together contribute to progressive impairment of cellular structure and function.[29]
Periodontitis is the most common oral infection in humans and the major cause of tooth loss in adults, has been considered a complication of diabetes.[30],[31] A large number of investigations have provided evidence that types 1 and 2 diabetes increase the risk and severity of periodontitis.[32],[33],[34], [35],[36],[37] The explanation for this is provided by a number of cellular and molecular alterations taking place in the periodontium as a consequence of sustained hyperglycemia.[38] Periodontitis has also been shown to have an impact on diabetes, although less evidence is available on the underlying mechanisms. The entrance of microorganisms and/or their products in the systemic circulation and the host inflammatory response are believed to play a role in this process.[39], [40], [41], [42]
In the present study probing depth and clinical attachment level were assessed to assess the periodontal destruction. Since many studies[43], [44], [45] have chosen probing depth and clinical attachment level as marker for periodontal destruction, same were chosen for the present study. Fasting blood sugar level is one of the reliable tests for the measuring degree of diabetes on that present day. And glycated hemoglobin level gives the history of glycemic control of three months. Hence both fasting blood sugar level and glycated hemoglobin levels were recorded to assess the glycemic control of diabetic population in the study.
Doxycycline is a family of tetracycline which has shown highest concentration in gingival crevicular fluid concentration.[46] Studies[47],[48] have shown the beneficial effect of doxycycline as adjunct to non-surgical therapy. Even though many different concentrations have been tried, doxycycline used in 100 mg per day for 14 days has been used as systemic antimicrobial dose in many studies.[49], [50], [51], [52]
Ryan et al.[53] reported a decrease in the level of glycated hemoglobin and collagen degradation in diabetic rats following administration of doxycycline or chemically-modified tetracycline. The authors hypothesized that extracellular glycation of proteins in diabetes is inhibited by tetracycline via a non-anticollagenase mechanism. Gingival crevicular fluid and salivary collagenases were also significantly inhibited following administration of systemic tetracycline in labile diabetics as well as in individuals with rheumatoid arthritis. Tetracyclines and their non-antimicrobial chemically-modified derivatives can 1) prevent oxidative activation of latent promatrix metalloproteinases, 2) down regulate matrix metalloproteinases expression and 3) protect the body's major serine proteinase inhibitor (elastase) from both oxidative and matrix metalloproteinase–dependent inactivation. Furthermore, protein synthesis and secretion by periodontal ligament fibroblasts was increased in diabetic rats following tetracycline administration. Overall, this evidence has provided the basis for a therapeutic approach to controlling periodontal disease in individuals with diabetes using tetracyclines and their derivatives.[23],[54],[55]
In the present study plaque and gingival inflammation were reduced after non-surgical periodontal therapy, but there was no difference between the groups. Also the subjects receiving doxycycline showed no difference in plaque index and gingival index scores compared to subjects not receiving doxycycline. This shows the effectiveness of the oral hygiene program adopted in this study. This is in accordance with study done by De Pommereau V et al. and Gabbriela Alessandra da Cruz et al.[56],[57]
In the present study, test and control groups showed improvement in the probing depth and clinical attachment level from baseline to 3 month follow up. The subjects receiving doxycycline had significant greater reduction in PD and CAL compared to subjects who did not receive doxycycline. Similar results have been demonstrated in studies done by Patricia et al.[58] and Goncalves D et al.[59]
Christgau et al.[13] found that probing pocket depth was reduced significantly in pocket with initial probing depth of ≥ 4mm which coincides with the present study, where average of initial probing depth of all groups were around 4 mm. The improvement in test group can be explained by the anti-microbial as well as anti-inflammatory action of doxycycline.
FBS reduced significantly in both test as well as control groups from baseline to 3 months follow up. FBS was also reduced significantly in test group when compared to control group. FBS reduced by a mean difference of 33.3 mg/dl in test group i.e subjects taking doxycycline. Similar results were shown in study done by Singh et al.[60] where they used 100 mg of doxycycline as adjunctive systemic anti-microbials with scaling and root planing. They found that there was more reduction in fasting glucose levels in group receiving doxycycline than compared to non-surgical therapy alone group, than compared to no treatment group. Patricia et al.[58] also showed that there was reduction in the fasting blood sugar level, but not statistically significant.
HbA1c was significantly reduced in after non-surgical therapy and more so when doxycycline was used. Singh et al.[60] had also assessed HbA1c levels were they found that there was reduction in HbA1c levels in group receiving doxycycline, more compared to group receiving only nonsurgical periodontal therapy. Similar results were obtained by Paricia et al.[58] and Grossi et al.[61]
Stewart et al.[15] reported a decrease in the levels of HbA1C following nonsurgical therapy of periodontitis in type 2 DM patients. They showed improvement in HbA1C levels in the control group. The authors suggest that this was possibly due to change in diabetic control in some patients. For this reason, in our study, we did not attempt to change the diabetic control of our patients by giving any additional instructions for control of blood glucose levels.
Conclusion
In the present study we can conclude that periodontal therapy with adjunct Doxycycline 100 mg systemically administered have a impact on metabolic control of type 2 DM, where both fasting blood sugar level as well as glycated hemoglobin levels have shown an improvement over 3 months. It has also shown that periodontal therapy alone also has a beneficial effect on metabolic control in diabetes mellitus individuals. Also there is significant reduction in probing dept and clinical attachment level in patients receiving scaling & root planing along with doxycycline 100mg systemic administration.
Reference
1. Brian L. Mealey & Gloria L. Ocampo: Diabetes mellitus and periodontal disease: Peridontology 2000 Vol. 44, 2007, 127-153.
2. Mealey B L.1996 World Workshop in Clinical Periodontics. Periodontal implication: medically compromised patients. Ann Periodontol 1996;1:256- 321
3. Papapanou PN.1996 World Workshop in Clincal Periodontics. Periodontal disease: Epi-demiology. Ann Periodontol 1996;1:1- 36.
4. Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and Periodontal disease: a case-controlled study. J Periodontol 2005;76:418-425.
5. Ervasti T, Knuutila M, Pohjamo L, Haukipuro. Relation between control of diabetes and gingival bleeding. J Periodontol 1985;56:154-157.
6. Gusberry F A, Sayed S A, Becon G, Grossman N, Loeshe W J. Puberty gingivitis in insulin dependent diabetic children. J Periodontol 1983;54:714-720.
7. Karjalainen K M, Knuutila M L E. The onset of diabetes and poor metabolic control increases gingival bleeding in children and adolescent with insulin dependent diabetes mellitus. J Clin Periodontol 1996;23:1060-1067.
8. Emrich L J, Shlossman M, Genco R J. Periodontal disease in non insulin dependent diabetes mellitus. J Periodontol 1991;62:123-130.
9. Sholssman M, Knowler W C, Pettitt D J, Genco R J. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc 1990;121:532-536.
10. Taylor GW, Burst B A, Becker M P, Genco R J, Shlossman M, Knowler W C, Pettitt D J: Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol 1998;69:76 – 83.
11. Taylor GW, Burst B A, Becker M P, Genco R J, Shlossman M, Knowler W C, Pettitt D J: Severe Periodontitis and Risk for poor glycemic control in patients with non-insulin dependent diabetes mellitus. J Periodontol 1996;67:1085 – 1093.
12. Sergio Guzman, Mamdouh karima, Hwa-Ying Wang, and Thomas E, Van dyke. Association between interleukin – 1 Genotype and Periodontal disease in a Diabetic Population. J Periodontol 2003;74:1183-1190.
13. Christgau M, Palitzch K D, Kreiner S.G. Frenzel S. Healing response to non-surgical periodontal therapy in diabetes mellitus clinical microbiological and immunological results. J Clin Periodontol 1998;25:112-124.
14. Westfelt E, Rylander H, Blohme G, Jonasson P, Lindhe J. The effect of periodontal therapy in diabetes. Results after 5 years. J Clin Periodontol 1996;23:92-100.
15. Stewart J E, Wager K A, Friedlander A H, Zadeh H. The effect periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol 2001;28:306-310.
16. G Alessandra Da Cruz, Sergio de Toledo, Enilson Antionio Sallum, A.W. Sallum, Glancia M.B. Ambrosano, J.D.C.O.sardi, S. Cruz, and R.B. Goncahus. Clinical and Laboratory Evaluation of Non-Surgical periodontal treatment in subjects with diabetes Mellitus. J Periodontol 2008;79:1150-1157.
17. Faria – Almedia R, Navarro A, Bascones A. Clinical and metabolic changes after conventional treatment of type 2 diabetes patients with chronic periodontitis. J Periodontol 2006;77:591-598.
18. Mauley B L: Influence of periodontal infection on systemic health. J Periodontol 2000;21:197-199.
19. Mauley G L: Diabetes Mellitus. In Rose L F, Genco R J Meuley B L et al, editors: Periodontal medicine, Toronto, 2000, B C Decker.
20. Grossi S G, Genco R J: Periodontal disease and diabetes mellitus: A two way relationship. Ann Periodontol 1998;3:51.
21. Herrera D, SanzM, Jepsen S, Needleman I, Roldan S : A systemic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol 2002;3:136-159.
22. Anne D Haffajee, S.S. Socransky, and John C Gunslky. Systemic Anti-infective Periodontal Therapy. A Systemic review. Ann Periodontol 2003;8:115-181.
23. Yoshihiro Iwomoto, Fusanori Nishimura, Masatsuger Nakagawa, Hikara Sugimota kenichi shikata, Hirofumi Makino, Tetsaya Fukuda, Takao Tsuji, Masohiro Iwomoto & Yoji Murayama. The effect antimicrobials periodontal treatment on circulating Tumour Necrosis Factor Alpha and Glycated Hemoglobin level in patients with type 2 diabetes. J Periodontol 2001;72:774-778.
24. Sergio Guzman, Mamdouh karima, Hwa-Ying Wang, and Thomas E, Van dyke. Association between interleukin – 1 Genotype and Periodontal disease in a Diabetic Population. J Periodontol 2003;74:1183-1190.
25. Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab 2005:7:307-317.
26. Diamond J. The double puzzle of diabetes. Nature 2003;423:599-602.
27. Marshall SM, Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. BMJ 2006;333:475-480.
28. Barr RG, Nathan DM, Meigs JB, Singer DE. Tests of glycemia for the diagnosis of type 2 diabetes melli-tus. Ann intern Med 2002:137:263-272.
29. Evanthia Lalla, Ira b. Lamster, Steven Drury, Caifeng Fu & Ann Marie Schmidt. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontology 2000; vol 23, 2000, 50-62.
30. Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993; 16: 329-334.
31. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366:1809-1820.
32. Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol 1991;62:123-131.
33. Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol 1994;65:260-267.
34. Hugoson A, Thorstensson H, Falk H, Kuylenstierna J. Periodontal conditions in insulin-dependent diabetics. J Clin Periodontol 1989;16:215-223.
35. Nelson RG, Shlossman M, Budding LM, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care 1990;13:836-840.
36. Oliver RC, Tervonen T. Periodontitis and tooth loss: Comparing diabetics with the general population. J Am Dent Assoc 1993;124:71-76.
37. Papapanou PN. Periodontal diseases: Epidemiology. Ann Periodontol 1996;1:1-36.
38. American Academy of Periodontology. Diabetes and periodontal diseases (position paper). J Periodontol 1996;67:166-176.
39. Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol 1996;67(Suppl.):1123-1137.
40. Grossi SG. Treatment of periodontal disease and control of diabetes: An assessment of the evidence and need for future research. Ann Periodontol 2001:6:138-145.
41. Page RC. The pathobiology of periodontal diseases may affect systemic diseases: Inversion of a paradigm. Ann Periodontol 1998;3:108-120.
42. Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Reo 2000;13:547-558.
43. B. Safkan Seppala and J. Ainamo: Periodontal Condition in insulin-dependent diabetes mellitus. J Clin Periodontol1992;19:24-29.
44. Seppala B, Seppale M and Ainamo J: A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol 1993;20:161-165.
45. Tervonen T and Oliver RC: Long term control of diabetes mellitus and periodontitis. J Clin Periodontol 1993;20:431-435.
46. Seymour RA, Heasman PA: Tetracycline in the management of periodontal disease. A Review. J Clin Periodontol 1995;22:22-35.
47. Matisko MW, Bissada NF. Short-term sequential administration of amoxicillin/clavulanate potassium and doxycycline in the treatment of recurrent/progressive periodontitis. J Periodontol 1993:64:553–558.
48. Bridge RB, Anderson JW, Saxe SR, Gregory K, Bridge SR. Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control, and socioeconomic factors. J Periodontol 1996;67:1185-1192.
49. Feres M, Haffajee AD, Goncalves C, Allard KA, Som S, Smith C, Goodson JM, Socransky SS. Systemic doxycycline administration in the treatment of periodontal infections. I. Effect on the subgingival microbiota. J Clin Periodontol 1999:26:775–783.
50. Loesche WJ, Giordano J, Soebren S, Hutchinson R, Rau CP, Walsh L, Schork MA. Nonsurgical treatment of patients with periodontal disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996:81:533–543.
51. Lundström Å, Johansson LÅ, Hamp SE. Effect of combined systemic antimicrobial therapy and mechanical plaque control in patients with recurrent periodontal disease. J Clin Periodontol 1984:11:321–330.
52. Mandell RL, Socransky SS. Microbiological and clinical effects of surgery plus doxycycline on juvenile periodontitis. J Periodontol 1988:59:373–379.
53. Ryan ME, Ramamurthy NS, Golub LM: Tetracyclines inhibit protein glycation in experimental diabetes. Adv Dent Res.; 1998:12(2):152-8.
54. Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res.2005;84:1154–59.
55. Schmidt AM, Weidman E, Lalla E. Advanced glycation end products (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodont Res. 1996;31:508–15.
56. De Pommereau V, Dargent-Pare C, Robert JJ and Brion M: periodontal status in insulin dependent diabetic adolescents. J Clin Periodontol 1992;19:628-632.
57. G Alessandra Da Cruz, Sergio de Toledo, Enilson Antionio Sallum, A.W. Sallum, Glancia M.B. Ambrosano, J.D.C.O.sardi, S. Cruz, and R.B. Goncahus. Clinical and Laboratory Evaluation of Non-Surgical periodontal treatment in subjects with diabetes Mellitus. J Periodontol 2008;79:1150-1157.
58. O'Connell PA, Taba M, Nomizo A, Foss Freitas MC, Suaid FA, Uyemura SA, Trevisan GL, Novaes AB, Souza SL, Palioto DB, Grisi MF. Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol 2008 May;79(5):774-83.
59. Gonclaves D, Correa FOB, Khalil NM, de Faria Oliveira OMM, Orrico SRP. The effect of non-surgical periodontal therapy on peroxidase activity in diabetic patients: a case-conntrol pilot study. J Clin Periodontol 2008;35:799-806.
60. Singh S, Kumar V, Kumar S, Subbappa A. The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Int J Diabetes Dev Ctries. 2008 Apr; 28(2): 38-44.
61. Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, Genco RJ. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol 1997;68(8):713-9.
|