Introduction
As the scientific community is seeking alternatives to conventional treatment, periodontal researchers have found that phytotherapeutic agents are advantageous for suppressing bacteria that lead to periodontal diseases. So, there is a pronounced interest in the development of phytotherapeutic agents for the periodontal needs and therapy.
The word "phytotherapy" is a Latin term. The latin prefix phyto stands for plant and is called phuton in Greek (previously, phuto). Therapy comes from the Latin word therapia, originally from Greek therapeia, from therapeuein which means to treat medically. In other words "phytotherapy", means treatment with herbal medicine. Phytotherapy mainly refers to use of medicinal plants or herbs to prevent or cure diseases or to improve health conditions.
Historical Background
Plants (herbs) and naturally derived products from plants (herbal supplements) have been used to treat health ailments and have been relied upon since the beginning of recorded human history. One of the oldest surviving medical document, the Egyptian Ebers Papyrus, dated around 1550 BC, contains herbal remedies for over 876 illnesses. Hippocrates, the "Father of Medicine", in his writings contains references to over 250 medicinal plants and herbs. The scientific evidence- based literature also supports the efficacy and safety of numerous herbs today (Cohan and Jacobsen, 2000).[1]
Phytotherapy relies more on phytonutrients with the discovery that many plant food components can not only modify physiological function but also aid in medical practices such as drug delivery. Also, several plants and plant parts have anti-inflammatory, antioxidant, antibacterial, astringent and other useful properties. [2]
Hence, this article is aimed at highlighting the properties of plants and plant derived products that can be used in the periodontal therapy .
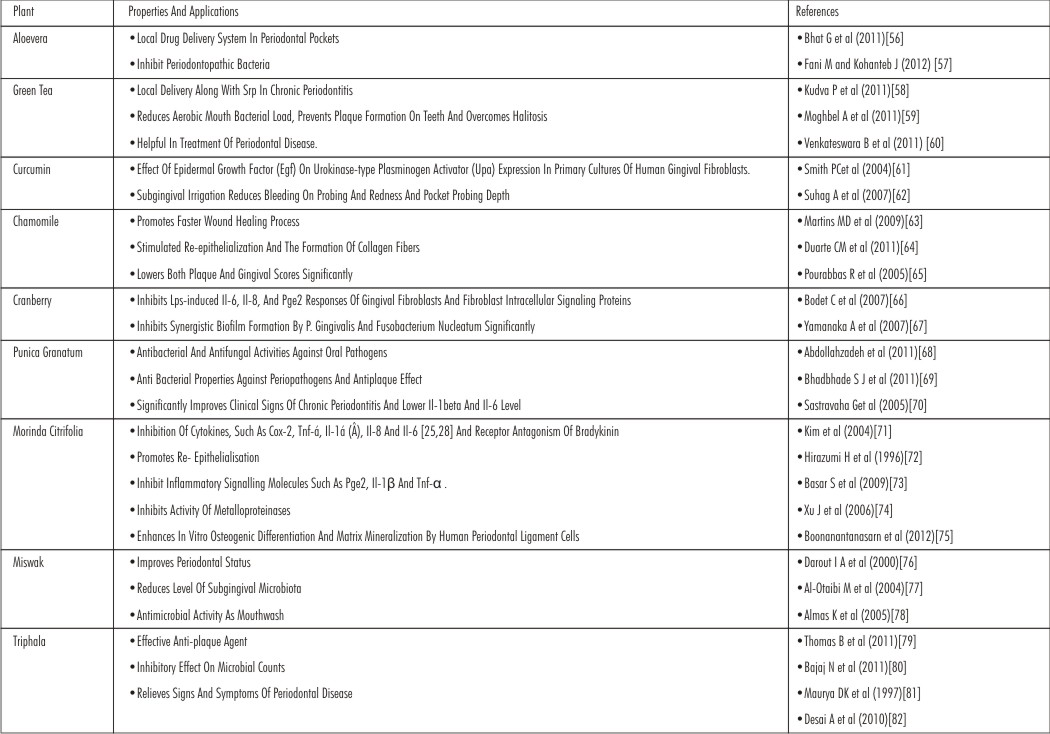
Aloevera
The Aloe vera that belongs to a Liliaceae family has been used for centuries for its medicinal and skin care properties. “Alloeh” meaning shining bitter substance, while “vera” in Latin means true. The Greek scientists regarded Aloe vera as the universal panacea.[3] The aloevera leaf consists of two different parts: central mucilaginous and peripheral bundle sheath cells. The parenchymal makes up the inner portion of the aloe leaves and produces a clear, thin tasteless jelly like material called Aloe vera gel.[4] Aloe vera contains 75 potentially active constituents: vitamins , enzymes , minerals , sugars , lignins , saponins , salicylic acids and amino acids.[5] Aloe vera is a potent anti-inflammatory agent and inhibits the cyclooxygenase pathway and reduces prostaglandin E2 production from arachidonic acid.[1] Aloe vera molecule synergizes with plant growth factors to repair and produce growth causing inhibition of pain and inflammation, stimulation of fibroblasts to functionally produce collagen & proteoglycans which increases wound tensile strength. It has proven its efficacy as anti inflammatory, antiviral, antibacterial and antioxidative and enhancing defence mechanisms increasing its use as a herbal remedy in periodontal disease and other oral conditions.
Green Tea
Green tea is one of the most popularly consumed beverage worldwide contains a number of bioactive chemicals and is particularly rich in flavonoids and also contains carotenoids, tocopherols, ascorbic acid (Vitamin C), minerals such as Cr, Mn, Se or Zn, and certain phytochemical compounds.[6],[7] Its healthful properties are largely attributed to polyphenols, chemicals with potent antioxidant properties. The antioxidant effects of polyphenols appear to be greater than those of vitamin C. Polyphenols contained in teas are classified as catechins. Green tea contains six primary catechin compounds: catechin, gallaocatechin, epicatechin, epigallocatechin, epicatechin gallate (Ecg), and epigallocatechin gallate (EGCg). EGCg is the most studied polyphenol component in green tea. The scavenging capacity of catechin and epicatechin molecules depends on their hydrogen donating ability. It is demonstrated that polyphenols have inhibitory effect on the ROS generation as well as on the release of lysosomal enzymes.[6] Numerous studies in a variety of experimental animal models have demonstrated that catechin possess antioxidant, antimutagenic, antidiabetic, anti-inflammatory, antibacterial and antiviral, and above all, cancer-preventive properties.[6],[7],[8]
Curcumin
Turmeric is a spice that contains the polyphenol curcumin in its rhizome. Turmeric is comprised of a group of three curcuminoids: curcumin (diferuloylmethane), demetho xycurcumin, and bisdemethoxycurcumin as well as volatile oils (tumerone, atlantone, and zingiberone), sugars, proteins, and resins. Curcumin modulates the inflammatory response by down-regulating the activity of cyclooxygenase-2 (COX-2), lipoxygenase, and inducible nitric oxide synthase (iNOS) enzymes; inhibits the production of the inflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin (IL) 1, 2, 6, 8, and 12, monocyte chemoattractant protein (MCP), and migration inhibitory protein; and down-regulates mitogen-activated and Janus kinases.[9],[10] In addition to inhibiting lipid peroxidation, curcumin demonstrates free radical-scavenging activity. Curcumin's reported properties include anti-inflammatory,[11],[12] anti-oxidant,[13] anti - bacterial,[14] and wound healing properties[15],[16] are desirable assets, which validate its use in the treatment of periodontitis.
Chamomile
M. chamomilla L. and M. suaveolens L.), of the Asteraceae family, is one of the tea ingredients most used worldwide. The whole plant is odoriferous and of value, but the quality is chiefly centred in the flower-heads or capitula, the part employed medicinally. The essential oil contains a-bisabolol (up to 50%) chamazulene cyclic sesquiterpenes,[17], [18], [19] which directly reduce inflammation and are mild antibacterials. Also, it contains bisabolol oxides, farnesene and spiro-ether, which have anti-inflammatory and antispasmodic actions. It has been used for centuries, particularly because of its anti-inflammatory, antimicrobial, antispasmodic and sedative effects.[20] The principal chemical components of the flowers include several phenolic compounds, primarily the flavonoids apigenin, quercetin, patuletin, luteolin and their glucosides. The main components of the essential oil extracted from the flowers are the terpenoids alpha-bisabolol and its oxides and azulenes, including chamazulene.[21], [22], [23], [24], [25]
Cranberry
Cranberry (Vaccinium macrocarpon) is a shrub that grows in the peat bogs of cold regions of northeastern North America. Cranberry extracts are particularly rich in polyphenols,[26] including flavanoids, which have biological properties that can be beneficial to human health.
Cranberries were also used to “clear the blood”, and as treatment for stomach ailments, liver problems, gall bladder disease, vomiting, appetite loss and scurvy.[27] Eastern Europeans adopted cranberry as a cancer remedy and antipyretic. Cranberry contains discrete flavonoids called condensed tannins, or proanthocyanidins (PACs), that exhibit unique microbial antiadhesion properties. Numerous studies have found that these PACs block uropathogenic bacteria from adhering to the uroepithelium and proliferating [28], [29], [30]. New evidence also suggests this same antiadhesion activity may also be helpful in the prevention of certain ulcers and periodontal disease.[31]
Punica Granatum
The pomegranate, Punica granatum L., an ancient, mystical, and highly distinctive fruit, is the predominant member of two species comprising the Punicaceae family. The pomegranate is native from the Himalayas in northern India to Iran but has been cultivated and naturalized since ancient times over the entire Mediterranean region. Pomegranate is used in several systems of medicine for a variety of ailments. In Ayurvedic medicine the pomegranate is considered “a pharmacy unto itself ” and is used as an antiparasitic agent,[32] a “blood tonic,”[33] and to heal aphthae, diarrhea, and ulcers.[34] Pomegranate also serves as a remedy for diabetes in the Unani system of medicine practiced in the Middle East and India.[35]
Current research seems to indicate the most therapeutically beneficial pomegranate constituents are ellagic acid ellagitannins (including punicalagins), punicic acid, flavonoids, anthocyanidins, anthocyanins, and estrogenic flavonols and flavones.[36], [37], [38], [39], [40]
An in vitro assay using four separate testing methods demonstrated pomegranate juice and seed extracts have 2-3 times the antioxidant capacity of either red wine or green tea.[41] Pomegranate extracts have been shown to scavenge free radicals and decrease macrophage oxidative stress and lipid peroxidation in animals[42] and increase plasma antioxidant capacity in elderly humans.[43] Cold pressed pomegranate seed oil has been shown to inhibit both cyclooxygenase and lipoxygenase enzymes in vitro. [38]
Numerous in vitro studies[44],[45] and two human trialsdemonstrate the antimicrobial activity of pomegranate extracts. The growth of Staphylococcus aureus, Streptococcus pyogenes, Diplococcus pneumoniae, Escherichia coli O157:H7, and Candida albicans was inhibited via direct bacteriocidal or fungicidal activity.
Morinda Citrifolia
Morinda citrifolia is one of the traditional folk medicinal plantsin Polynesia, and Southeast Asia, also known as noni. The major components of the whole noni plant have been found such as scopoletin, octoanoic acid, potassium, ascorbic acid (vitamin C), terpenoids, alkaloids, anthraquinones, β-sitosterol, carotene, retinoic acid (vitamin A), flavone glycosides, linoleic acid and amino acids, including calcium and phosphorus.[46],[47] Noni has been reported for a broad range of usage including antibacterial, antiviral, antifungal, antitumor, antihelmin, analgesic, hypotensive, anti-inflammatory, and immune enhancing effects. [46],[48], [49], [50]
The primary usage of noni is to apply leaves as a traditional topical treatment thought to enhance wound healing. Noni leaf extract was capable of promoting wound healing in an animal model. [51] In addition, the crude extract of noni leaf has been traditionally used in patients with bone fractures or dislocation to promote tissue repair and decrease inflammation. [46] It is well established that bone and periodontal tissue repair or regeneration requires growth factors to induce progenitor/precursor cells to differentiate and produce matrix mineralization. [52]
Miswak
Pencil-sized sticks of various plants are fashioned from certain plant - parts and are chewed on one end until they become frayed into a brush. The brush-end is used to clean the teeth in a manner similar to the use of a toothbrush. When used in this manner, they are commonly referred to as chewing sticks or Miswak. The conventional meaning of Miswak is 'stick used on teeth and gums to clean them.' Farooqi et al(1968)[53] isolated benzy-lisothiocyanate from Salvadora persica root, they claimed to have found saponins along with tannins, silica, a small amount of resin, trimethylamine , a fairly large amount of alkaloidal constituents, B-sitosterol, m-anisic acid, salvadourea [1,3-Bis-(3-methoxy-benzyl)-urea] and a high content of minerals in the root: 27.06%.
Triphala
Triphala is a tridoshic formula of fruits of Terminalia belerica (Family: Combretaceae), Terminalia chebula (Family: Combretaceae) and Emblica officinalis (Family: Euphorbiaceae).It is considered a nourishing, balancing and rejuvenating formula that helps the body to detoxify and supports the digestive action. Its phytochemical constituents are tannin, gallic acid, chebulagic acid, ellagic acid, phenols, glycosides and flavonoids. Conditions for which Triphala is employed include headache, dyspepsia, constipation, liver conditions, ascites and leucorrhoea. It is also used as a blood purifier that can improve the mental faculties and it posseses antiinflammatory, analgesic, anti-arthritic, hypoglycemic and anti-aging properties.[54],[55]
 |
 |
Summary & Conclusion
Phytotherapy, the most ancient medication, is still useful today. Some unstudied plants may still have hidden secrets for the medical world. However, concerns from scientists, professionals and customers continuously arise, due to increases in the use of phytochemicals. Quality, safety, long-term adverse effects and toxicity are the primary concerns.
The scientific evidence supporting their use requires closer scrutiny and expansion before it can be fully accepted as part of everyday practice. Even though not every traditional custom has been scientifically validated, they need not be summarily dismissed as quackery. Proponents of both modern and traditional medicine need to shed long-held beliefs and accept existing evidence before such practices can be truly integrated into present-day periodontal therapy.
As demonstrated by the examples included in this Literature review, there is considerable evidence that plant extracts, essential oils and purified phytochemicals have the potential to be developed into agents that can be used as preventive or treatment therapies for oral diseases. While it is encouraging to see a number of clinical trials of such products, further studies of the safety and efficacy of these agents will be important to establish whether they offer therapeutic benefits, either alone or in combination with conventional therapies, that can help to reduce the overall burden of oral diseases worldwide.
References
1. Cohan, R.P. and P.L. Jacobsen, 2000. Herbal supplements: Considerations in dental practice. J. California Den. Assoc., 28: 600-6010.
2. Bland JS. Phytonutrition, phytotherapy, and phytopharmacology. Altern Ther Health Med 1996; 2:73-76.
3. Sikarwar MS, Patil MB, Sharma S, Bhat V. Aloe vera: Plant of Immortality. International Journal of Pharma Sciences and Research 2010; 1(1): 7-10.
4. Wynn RL. Aloe vera gel: Update for dentistry. Gen Dent. 2005;53:6–9.
5. Atherton P Nurs Stand. Aloe vera: magic or medicine 1998 7;12(41):49-52, 54.
6. Mckey DL, Blumberg JB. The role of tea in human health: Anupdate. J Am Coll Nutr 2002;21:1-13.
7. Wiseman SA, Balentine DA, Frei B. Antioxidants in tea. Crit Rev Food Sci Nutr 1997;37:705-18.
8. Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol 1997;24:287R09;96.
9. Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “curecumin”: from kitchen to clinic. Biochem Pharmacol 2008;75:787-809.
10. Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res 1999;39:41-47.
11. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric. J Altern Complement Med 2003;9:161–168.
12. Ramsewak RS, DeWitt DL, Nair MG. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine 2000;7:303–308.
13. Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of the tetrahydrocurcuminoids. Biosci Biotechnol Biochem 1995;59:1609–1612.
14. Singh R, Chandra R, Bose M, Luthra PM. Antibacterial activity of Curcuma longa rhizome extract on pathogenic bacteria. Curr Sci 2002;83:737–740.
15. Sidhu GS, Singh AK. Enhancement of wound healing by curcumin in animals. Wound Repair Regen 1998;6:167–177.
16. Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma 2001;51:927–931.
17. Grieve M. A modern herbal. Dover Publications Inc. 1982 The Merck Index. 12th ed. Merck Research Labs. Division of Merck and Co. Whitehouse Station, NJ. 1996:4434.
18. Petri G and Lemberkovics E. Gyogynovenyek es drogjaik muszeres vizsgalatanak lehetosegei. Acta Pharmaceutica Hungarica. 1994;64:87-93.
19. Hoffmann D. Therapeutic herbalism. A correspondence course in phytotherapy. 1995(4)30-31.
20. O’Hara M, Kiefer D, Farrell K, Kemper K. A review of 12 com¬monly used medicinal herbs. Arch Fam Med. 1998;7:523-36.
21. World Health Organization. Monographs on selected me¬dicinal plants. v.1. Geneva (Switzerland): WHO; 1999. [cit¬ed 2009 Jun 12]; Available from: http://whqlibdoc.who.int/ publications/2002/9241545178.pdf.
22. Jakovlev V, Isaac O, Thiemer K, Kunde R. [Pharmacological in¬vestigations with compounds of chamomile ii. new investigations on the antiphlogistic effects of (-)-alpha-bisabolol and bisabolol oxides (author’s transl)]. Planta Med. 1979;35:125-40.
23. Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol. 2000;59:1387-94.
24. Szoke E, Máday E, Tyihák E, Kuzovkina IN, Lemberkovics E. New terpenoids in cultivated and wild chamomile (in vivo and in vitro). J Chromatogr B Analyt Technol Biomed Life Sci. 2004;800:231-8.
25. McKay DL, Blumberg JB. A review of the bioactivity and poten¬tial health benefits of chamomile tea (Matricaria recutita L.). Phy¬tother Res. 2006;20:519-30.
26. Vvedenskaya IO, Rosen RT, Guido JE, Russell DJ, Mills KA, Vorsa N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J Agric Food Chem. 2004;52(2):188-95.
27. Siciliano A. Cranberry. HerbalGram 1996; 38:51-54.
28. Howell AB, Botto H, Combescure C, Blanc-Potard A-B, Gausa L, Matsumoto T, Tenke P, Sotto A, Lavigne JP. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94.
29. Howell AB, Foxman B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA. 2002;287(23):3082-3.
30. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.
31. Weiss, E.I., Lev-Dor, R., Kashmamn, Y., Goldhar, J., Sharon, N., Ofek, I. Inhibiting interspecies coaggregation of plaque bacteria with a cranberry juice contrituent. J. Am. Dent. Assoc. 1998;129:1719-1723.
32. Naqvi SA, Khan MS, Vohora SB. Antibacterial, antifungal, and antihelminthic investigations on Indian medicinal plants. Fitoterapia 1991;62:221-228.
33. Lad V, Frawley D. The Yoga of Herbs. Santa Fe, NM: Lotus Press; 1986:135-136.
34. Caceres A, Giron LM, Alvarado SR, Torres MF. Screening of antimicrobial activity of plants popularly used in Guatemala for treatment of dermatomucosal diseases. J Ethnopharmacol 1987;20:223-237.
35. Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: a review. J Altern Complement Med 2004;10:369-378.
36. Du CT, Wang PL, Francis FJ. Anthocyanins of pomegranate, Punica granatum. J Food Sci 1975;40:417-418.
37. Artik N. Determination of phenolic compounds in pomegranate juice by using HPLC. Fruit Processing 1998;8:492-499.
38. Schubert SY, Lansky EP 14. , Neeman I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J Ethnopharmacol 1999;66:11-17.
39. Abd El Wahab SM, El Fiki NM, Mostafa SF, et al. Characterization of certain steroid hormones in Punica granatum L. seeds. Bull Fac Pharm 1998;36:11-15.
40. Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: dephinidin, cyanidin, and pelargonidin. J Agric Food Chem 2002;50:166-171.
41. Gil MI, Tomas-Barberan FA, Hess-Pierce B, et al. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 2000;48:4581-4589.
42. Rosenblat M, Volkova N, Coleman R, Aviram M. Pomegranate byproduct administration to apolipoprotein e-deficient mice attenuates atherosclerosis development as a result of decreased macrophage oxidative stress and reduced cellular uptake of oxidized low-density lipoprotein. J Agric Food Chem 2006;54:1928-1935.
43. Guo C, Wei J, Yang J, et al. Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutr Res 2008;28:72-77.
44. Voravuthikunchai SP, Limsuwan S. Medicinal plant extracts as anti-Escherichia coli O157:H7 agents and their effects on bacterial cell aggregation. J Food Prot 2006;69:2336-2341.
45. Braga LC, Shupp JW, Cummings C, et al. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol 2005;96:335-339.
46. Wang MY, West BJ, Jensen CJ, Nowicki D, Su C, et al. (2002) Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol Sin 23: 1127-1141.
47. Potterat O, Hamburger M (2007) Morinda citrifolia (Noni) fruit--phytochemistry, pharmacology, safety. Planta Med 73: 191-199.
48. Deng S, Palu K, West BJ, Su CX, Zhou BN, et al. (2007) Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. J Nat Prod 70: 859-862.
49. Hirazumi A, Furusawa E, Chou SC, Hokama Y (1996) Immunomodulation contributes to the anticancer activity of morinda citrifolia (noni) fruit juice. Proc West Pharmacol Soc 39: 7-9.
50. Akihisa T, Nakamura Y, Tagata M, Tokuda H, Yasukawa K, et al. (2007) Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem Biodivers 4: 224-231.
51. Nayak BS, Sandiford S, Maxwell A (2009) Evaluation of the Wound-healing Activity of Ethanolic Extract of Morinda citrifolia L. Leaf. Evid Based Complement Alternat Med 6: 351-356.
52. Taba M Jr, Jin Q, Sugai JV, Giannobile WV (2005) Current concepts in periodontal bioengineering. Orthod Craniofac Res 8: 292-302.
53. Farooqi MIH and Srivastava JG. The toothbrush tree (Salvadora perslca). Quart. J. Crude Drug Res. 1968;8:1297-99.
54. Mehta BK, Shitut S, Wankhade H. In vitro antimicrobial efficiency of triphala. Fitoterapia 1993; 64: 371-372.
55. Vani T, Rajani M, Sarkar S, Shishoo CJ. Antioxidant properties of the Ayurvedic formulation Triphala and its constituents. Int J Pharmacogn. 1997; 35:313-317.
56. BhatG, KudvaP, and DodwadV. Aloe vera: Nature's soothing healer to periodontal diseaseJ Indian Soc Periodontol. 2011 Jul-Sep; 15(3): 205–209.
57. Fani M and Kohanteb J.Inhibitory activity of Aloe vera gel on some clinically isolated cariogenic and periodontopathic bacteria.Journal of Oral Science, Vol. 54, No. 1, 15-21, 2012.
58. Kudva P, Tabasum S T, Shekhawat N K. Effect of green tea catechin, a local drug delivery system as an adjunct to scaling and root planning in chronic periodontitis patients: A clinicomicrobiological study.Journal of Indian Society of Periodontology - Vol 15, Issue 1, Jan-Mar 2011.
59. Moghbel A, Farjzadeh A, Aghel N , Agheli H , Raisi N.The Effect of Green Tea on Prevention of Mouth Bacterial Infection, Halitosis, and Plaque Formation on Teeth.Iranian Journal of Toxicology- Volume 5, No 14, Autumn 2011.
60. Venkateswara B, Sirisha K, Chava VK. Reen tea extract for periodontal healh. J Indian Soc Periodontol 2011;15:18-22.
61. Smith PC, Santiban˜ez JF, Morales JP, Martinez J. Epidermal growth factor stimulates urokinase-type plasminogen activator expression in human gingival fibroblasts. Possible modulation by genistein and curcumin. J Periodont Res 2004; 39; 380–387.
62. Suhag A, Dixit J, Dhan P. Role of curcumin as a subgingival irrigant: a pilot study. Quintessence: Perio 2007;4(2):115–121
63. Martins MD, Marques MM, Bussadori SK, Martins MA, Pavesi VC, Mesquita-Ferrari RA, Fernandes KP.Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother Res. 2009 Feb;23(2):274-8.
64. Duarte CM, Quirino MR, Patrocínio MC, Anbinder AL. Effects of Chamomilla recutita (L.) on oral wound healing in rats. Med Oral Patol Oral Cir Bucal. 2011 Sep 1;16 (6):e716-21.
65. Reza Pourabbasa, Abbas Delazarand Mohammad Taghi ChitsazThe Effect of German Chamomile Mouthwash on Dental Plaque and Gingival InflammationIranian Journal of Pharmaceutical Research (2005) 2: 105-109
66. Bodet C, Chandad F, Grenier D. Cranberry components inhibit interleukin-6, interleukin-8, and prostaglandin E2 production by lipopolysaccharide-activated gingival fibroblasts. Eur J Oral Sci 2007; 115: 64–70.
67. Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodont Res 2007; 42: 589–592.
68. Sh. Abdollahzadeh 1, 2, RY. Mashouf 3, H. Mortazavi 1, 2, MH. Moghaddam 4, N. Roozbahani 5, M. Vahedi Antibacterial and Antifungal Activities of Punica Granatum Peel Extracts Against Oral Pathogens.Journal of Dentistry, Tehran University of Medical Sciences, Tehran, Iran (2011; Vol. 8, No.1)
69. Smruti J. Bhadbhade, BDS1/Anirudh B. Acharya, MDS2/ Silvia V. Rodrigues, MDS3/Srinath L. Thakur, MDS4.The antiplaque efficacy of pomegranate Mouthrinse. Quintessence international,volume 42,Number 1,January 2011
70. Sastravaha G, Yotnuengnit P, Booncong P, Sangtherapitikul P. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts. A preliminary study. J Int Acad Periodontol 2003;5:106-115.
71. Kim, H.J., Jang, S.I., Kim, Y.J., et al. (2004) Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line, RAW 264.7 cells. Fitoterapia, 75, 261-266.
72. Hirazumi, A., Furusawa, E., Chou, S.C., et al. (1996) Im- munomodulation contributes to the anticancer activity of Morinda citrifolia L. (noni) fruit juice. Proceedings of the Western Pharmacology Society, 39, 7-9.
73. Basar, S., Uhlenhut, K., Westendorf, J., et al. (2009) An- algesic and anti-inflammatory activity of Morinda citrifo- lia L. (noni) fruit. Phytotherapy Research, 24, 38-42.
74. Xu, J., Mc Sloy, A.C., Anderson, B.K., et al. (2006) Tahi- tian Noni® equine essentialsTM: A novel anti-inflamma- tory and a COX-2 inhibitor which regulates LPS-induced inflammatory mediator expression in equine neonatal mo- nocytes. Journal of Veterinary Internal Medicine, 20, 756.
75. Boonanantanasarn K, Janebodin K, Suppakpatana P, Arayapisit T, Chunhabundit P, et al. (2012) Morinda citrifolia Leaf Enhances In Vitro Osteogenic Differentiation and Matrix Mineralization by Human Periodontal Ligament Cells. Dentistry 2:130.
76. Darout I A, Albandar J M. and Skaug N. Periodontal Status Of Adult Sudanese Habitual Users Of Miswak Chewing Sticks Or Toothbrushes.Acta Odontol Scand 58 (2000).
77. Al-Otaibi M, Al-Harthy M, Gustafsson A, Johansson A, Claesson R, Angmar-Mansson B: Subgingival plaque microbiota in Saudi Arabians after use of miswak chewing stick and toothbrush. J Clin Periodontol 2004; 31: 1048–1053.
78. Almas K, Skaug N, Ahmad I: An in vitro antimicrobial comparison of miswak extract with commercially available non-alcohol mouthrinses. Int J Dent Hygiene 3, 2005; 18–24.
79. Thomas B, Shetty Y S, Vasudeva A, Shetty V.Comparative evaluation of Antimicrobial Activity of Triphala and commercially available Toothpastes: An in-vitro study.International Journal of Public Health Dentistry 2011:2(1): 8-12.
80. Bajaj N and Tandon S. The effect of Triphala and Chlorhexidine mouthwash on dental plaque, gingival inflammation, and microbial growth.Int J Ayurveda Res. 2011 Jan-Mar; 2(1): 29–36.
81. D.K Maurya, N. Mittal, K.R. Sharma And G.Nath. Role Of Triphala In The Management Of Periodontal Disease.Ancient Science Of Life Vol No. 17 (2), October 1997.
82. Desai A, Anil M, Debnath S. A clinical trial to evaluate the effects of triphala as a mouthwash in comparison with chlorhexidine in chronic generalized periodontitis patient. IJDA, 2(3), July-September, 2010.
|