Introduction
Acrylic resins have been used for denture fabrication over six decades. The most popular denture base resin material is heat cured poly (methyl methacrylate) due to its many advantages such as dimensional stability, handling characteristics, ease of processing, capacity to mold in complex forms with application of pressure and heat.During the last years, curing processes have been modified in order to improve the physical and mechanical properties of those materials, and also to afford the technical work of the professionals. Different polymerization methods have been used are heat, light and microwave energy.[1], [2] The latter method has the advantage of reduced curing time, less duration for obtaining plastic phase, a bigger homogeneity of the mixture and excellent adaptation.Despite the above mentioned methods to polymerize denture base materials, the conversion of monomers to polymers is never complete and some unreacted monomers called residual monomers are left in the denture base polymers.[3]
Residual monomer presence has an adverse effect on physical and mechanical properties as well as on the biocompatibility.[4], [5], [6] Methyl methacrylate has the potential to elicit irritation, inflammation and allergic response of the oral mucosa.Further, it is capable of producing both stomatitis and an angular cheilitis.[7] Blagojevic and Murphy[8] reported that the residual monomer content of an autopolymerizing acrylic resin was reduced by nearly a quarter after microwaving. In addition, to minimize the amount of residual monomer released from denture following completion of polymerization, several authors have suggested that the prostheses should be stored in water prior to placement.[9] Tsuchiya H et[10] observed that the preleaching in water for 60 min at 50°C reduced the subsequent release of methyl methacrylate and formaldehyde, which decreased their cytotoxic potential .The aim of the present study is to investigate the effect of different conditions of microwave heating and conventional heating in water bath on the residual monomer levels of an acrylic resin denture base materials.
Materials & Methods
This in vitro study was conducted to evaluate the amount of residual monomer in 24,48,72,96 and 120 hrs period of time in acrylic resin samples of size 10×10×3 mm, processed with microwave polymerization method and compared with conventional heat cure polymerization method. Putty indices (EXAFLEX PUTTY GC AMERICA INC.) of 20 in number were prepared from the standard size 10×10×3mm metallic mold. Ten putty indices were invested with dental stone type III in Brass metal flask (VARSITY FLASK - JABBAR COMPANY ,National Dental Supply : New Delhi ) for heat cure polymerization and other tenin non metallic flask (MUFLA-VIPI-STG) (Fig: 1) for microwave polymerization. After the dental stone was completely set, the two portions of both brass flask and non metallic flask was opened carefully. Putty indices were recovered from the lower portion. Mold separator was carefully applied to all mold spaces for both brass and non metallic flasks. Heat cure material (DPI-heat cure: Dental Products Of India LTD, Mumbai) was mixed in proportion of 30: 10 ml by volume in the porcelain mixing jar. During dough stage the material was packed and trial closure was performed to eliminate excess flash.
 | Fig. 1. Non Metallic Flask For Microwave Polymerization
 |
Metallic flask was placed in water bath (DELTA-POLY BATH, DELTA BOILERS PVT,LTD: Mumbai) for polymerization. The resin samples were cured at 74°c for 2 hr and terminal curing done at 100°c for1 hr and then bench cooled. Acrylic specimens were recovered, without damaging specimen surfaces and dimensions (Fig :2). Nonmetallic flask was placed in the microwave oven (SAMSUNG-MW–73V) and cured at 450 watts for 2 min and then the flask was turned upside down and again cured at 450 watts for 2 min and flask was allowed to cool to the room temperature for 1 hr.
 | Fig. 2. Acrylic Resin Samples
 |
Residual Monomer Evaluation
Residual monomer content was determined by using a Double beam spectrophotometer (UV-Vis Spectrophotometer 2201, SYSTRONICS INDIA LTD, Ahmadabad) (Fig: 3). A spectrophotometer measures the amount of the light absorbed by a compound when it is subjected to the UV and visible spectrum (200-800nm) . The principle behind the Double beam spectrophotometer is that when light pass through a liquid substance, some of light gets absorbed and by measuring this, the concentration of that liquid substance can be analyzed. The amount of absorption was depends on the concentration of that substance. A stock solution of 1 % v/v methyl methacrylate was prepared by dissolving 1 ml of methyl methacrylate in 99 ml of distilled water. From the above stock solution a series of concentrations were prepared between 0.005 % to 0.8% by diluting with distilled water. By using Double beam spectroscopy at 210 nm the absorbencies of these standard solutions were determined and a standard graph was plotted between concentration and absorbance. Each specimen immediately after preparation was placed in screw capped containers consists of 20 ml of distilled water and stored at room temperature for 24,48,72,96 and 120 hrs periods of time. The solutions were transferred into the light transparent cuvets and they were subjected to double beam spectroscopy at 210 nm. The unknown amount of residual monomer leached into the distilled water was analyzed and values were compared with standard graph(Fig. 4).The results were expressed as a percent of released residual monomer mass with respect to the weight of the specimen (% w/w).[11]
 | Fig. 3. Double Beam Spectrophotometer Connected To The Monitor
 |
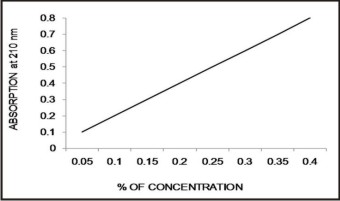 | Fig. 4. Standard Graph Of Mma Was Plotted Between Concentration And Absorbance.
 |
Results
The measured values are subjected to statistical analysis by using “unpairedt test” to know any significant difference between the two variables. The ‘mean’, ‘standard deviation’ and ‘p’ values are calculated for the variables. In this present study p < 0.05 is considered as the level of significance. The table shows the mean values in between the two processing methods and shows ‘p’ value is highly statistically significant. This indicates that, the residual monomer in microwave processing method has higher concentration than heat cure processing method.
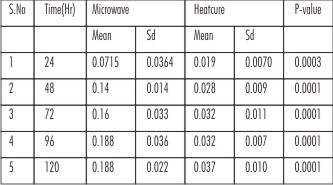 | Table:Comparison Of Mean And Standard Deviation Values Of Residual Monomer Analysis Between Microwave And Heat Cure Processing Techniques.
 |
Discussion
Inspite of many improvements in the material science and techniques, the conversion of monomer to polymer is never complete and some unreacted monomers called residual monomers are left in the denture base polymers. This left out residual monomer has proved to be cytotoxic causing erythema, erosion of mucous membrane, burning sensation of tongue and oral mucosa.[12] This residual monomer in the polymerized acrylic resins depends upon the chemical composition, the polymer to monomer ratio, the manipulative variables and storage in water. Polymerization with microwave energy was introduced as early as in 1968 by Nischii. In 1984 the fiber reinforced dental flasks are substituted with the brass flasks and water bath is replaced by microwave oven for microwave polymerization method. The uniform heating on either side of the flask and rapid raise of temperature with the resultant shortage of polymerization time makes microwave curing methods superior to the conventional water bath curing methods. The microwave heating is independent of thermal conductivity, so it leads to a curing cycle without the production of any unwanted exothermic heat.[13]
In this Spectrophotometric study the residual monomer content was high (0.0715) in microwave polymerized specimens when compared to heat cure specimens (0.019), the reason could be in microwave curing method, the monomer molecules vibrate by being exposed to a high frequency electromagnetic field. This phenomenon leads to intermolecular collisions that generate the heat for activation process and because of high power applied to the system, the benzoyl peroxide decomposition and polymerization reaction becomes faster. A rapid polymer structure hinders the conversion of MMA monomers especially at curing temperatures lower than the glass transition temperature (Tg) of polymer. Whereas the residual monomer concentration in heat cured denture base polymers was considerably lower than microwave polymers it was because of the higher the curing temperature, which can be as high as glass transition temperature (Tg) of the matrix phase of heat cured denture base polymer (97°c to 100°c). Above the Tg of the polymer the monomers of the resins have a better ability to polymerize due to higher molecular chain motions and neutralization of the immobilization of methyl methacrylate in the glassy polymer at higher temperature.[12] In this present study within different time intervals, heat cure processed samples showed maximum increase of residual monomer concentration in first 24, 48 and 72 hrs and then these values remain constant after 96 and 120hrs.Where as in microwave processed samples has shown increase in residual monomer concentration in first 24,48,72 and 96 hrs and then remains constant after 120 hrs. The decrease in the daily release of monomer occurred as a result of the monomer diffusion in water and by continuous polymerization promoted by the active radicals found in the polymer chains.
Conclusion
The results of residual monomer content analysis of acrylic resin samples processed through conventional heat cure and microwave curing methods at 24,48,72,96 and 120 hrs period of time has shown that there was a high statistically significant difference between these two processing methods. The residual monomer content of microwave specimens has shown higher values than heat cure specimens for the above said period of time. The residual monomer analysis at different time intervals has shown that the amount of leached residual monomer into the distilled water was higher in first 48 hrs (0.14 for microwave & 0.028 for heat cure resin samples) water storage and later it was greatly decreased.
Clinical significance: Spectrophotometric analysis is the standard method to analise the residual monomer concentration in acrylic resin samples. With this present study we can assess the cytotoxic potential of residual monomer present in various acrylic resins and method of reducing monomer concentration for resin samples, like water storage for more than 48 hr can be followed in clinical practice.
References
1. L.T. Smith, J.M. Powers and D. Ladd. Mechanical properties of new denture resins polymerized by visible light, heat and microwave energy.International Journal of Prosthodontics 1992;5: 315–320.
2. B. Levin, J.L. Sanders and P.V. Reitz. The use of microwave for processing acrylic resins. Journal of Prosthetic Dentistry 1989;61: 381–383.
3. J. A. Bartoloni, D. F. Murchison, D. T. Wofford & N. K. Sarkar. Degree of conversion in denture base materials for varied Polymerization techniques. Journal of Oral Rehabilitation 2000; 27: 488–493.
4. N. Yunus, A. Harrison and R. Hugget, Effect of microwave irradiation on the flexural strength and residual monomer levels of an acrylic resin repair material. Journal of Oral Rehabilitation 1994;21: 641–648.
5. A. Harrison, E.L. Belton and K. Meades, Do self-curing acrylic resin repairs gain strength with age?. Journal of Dentistry 1977;5: 334–338.
6. D.J. Lamb, B. Ellis and D. Priestly, The effect of process variable on levels of residual monomer in autopolymerizing dental acrylic resin. Journal of Dentistry 1983;11: 80–88.
7. Filiz A. Keyf, DDS, PhD, A. Ihsan Keyf, MD. Harmful effects of methylmethacrylate and formaldehyde from acrylic resin denture base materials. The Saudi Dental Journal 1998;10:23-28.
8. Blagojevic V, Murphy VM. Microwave polymerization of denture base materials. A comparative study. J Oral Rehabil. 1999;26:804-8.
9. Schuster GS, Lefebvre CA, Dirksen TR, Knoernschild KL, Caughman GB. Relationships between denture base resin cytotoxicity and cell lipid metabolism. Int J Prosthodont. 1995;8:580-6.
10. Tsuchiya H, Hoshino Y, Tajima K, Takagi N. Leaching and cytotoxicity of formaldehyde and methyl methacrylate from acrylic resin denture base materials. J Prosthet Dent. 1994;71:618-24.
11. M. J. Azzarri, M. S. Cortizoand J. L. Alessandrini .Effect of the curing conditions on the properties of an acrylic denture base resin microwave-polymerised. Journal of Dentistry 2003;31(7):463-468.
12. Janaina Habib Jorge, DDS, Eunice Teresinha Giampaolo, DDS, PhD, Ana Lucia Machado, DDS, PhD and Carlos Eduardo Vergani, DDS, PhD .Cytotoxicity of denture base acrylic resins. A literature review. J Prosthet Dent 2003; 90:190-3.
13. C.P. Laia, M.H. Tsaia, M. Chena, H.S. Changb, H.H. Tayb .Morphology and properties of denture acrylic resins cured by microwave energy and conventional waterbath. Dental Materials 2004; 20: 133–141.
|