Introduction
Leukemia is a group of malignant diseases of the hematopoietic system characterized by the uncontrolled overproduction of either immature or terminally differentiated leukocytes[1]. The initiation of leukemia occurs with the malignant transformation of one of the stem cells, which first proliferates in the bone marrow and then passes into the peripheral blood of the patient[2]. Acute leukemia, the most common form of cancer in children, accounts for approximately 30% of all childhood malignancies. As there are lot of advances in the therapeutic agents and chemotherapy, the subtyping of leukemia is very important. Morphology still remains one of the main techniques for establishing the diagnosis and classifying leukemia. On the basis of types of cells involved leukemias are classified into myeloid and lymphoid leukemias. A frequency of > 20 blasts is required to confirm a diagnosis of acute leukemia in the peripheral blood smear and / bone marrow[1]. Sub-typing leukemia using FAB (French-American-British) criteria recommended by the FAB group for classification makes the sub-classification easier to apply[3]. Our report describes a case of acute myeloid leukemia with oral manifestations in a 3 year old male child diagnosed using the peripheral blood smear.
Case Report
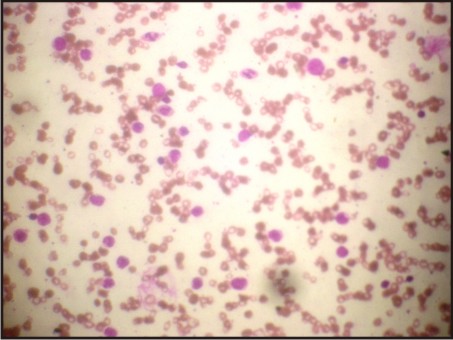
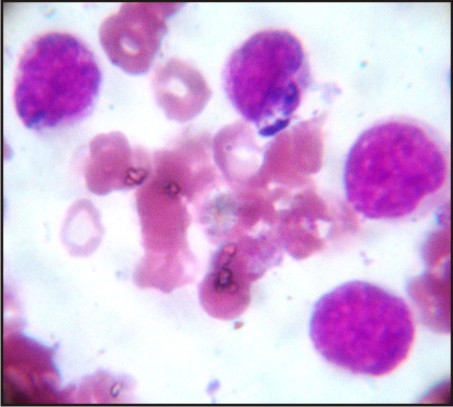
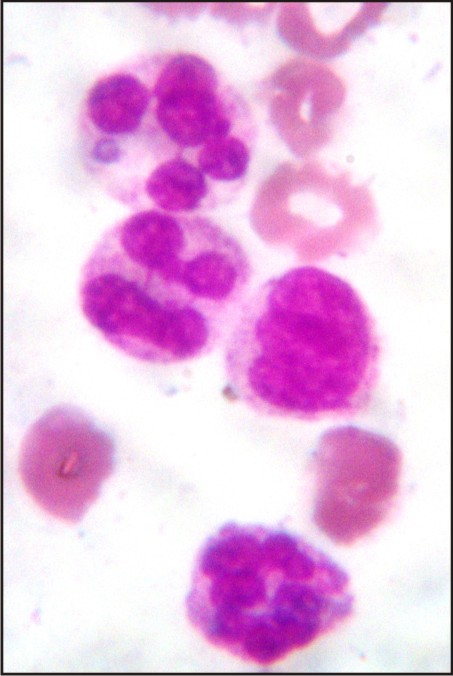
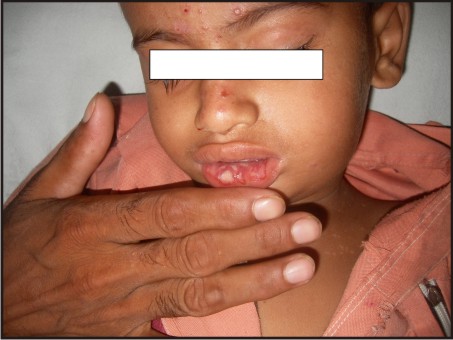
A 3 year old male patient was presented to the Department of Oral Medicine and Radiology, Subharti Dental College, Meerut, with a complaint of multiple ulcers in the mouth since 1 month. He had a past history of intermittent fever since 1 year and gastric upset since 2 years, for which he was on medication. On extraoral examination reddish blisters were seen over the nose and forehead (Fig 1). Submandibular lymph nodes were palpable bilaterally which were firm in consistency, mobile and tender. Also cervical lymph nodes were palpable, multiple in number and tender. On intraoral examination generalized diffuse gingival enlargement surrounding all the teeth was seen (Fig 2). Multiple painful ulcers were present with erythematous area surrounding the necrotic halo, 2mm in size on the tongue and ulcers filled with whitish slough, on the labial mucobuccal fold (Fig 2) which led to the provisional diagnosis of cyclic neutropenia, leukemia and non hodgkins lymphoma. A routine hemogram was performed in the Department of Oral pathology which revealed hemoglobin of 6.7g %, with leukocytosis (total leucocyte count of 1,10,000/cumm)and thrombocytopenia (platelet count 1,00,000 /mm3). The peripheral blood smear showed, leukoerythroblastic picture with blasts being the predominant cells (Fig 3). These blasts were large cells with increased nucleocytoplasmic ratio, finely dispersed chromatin and scant to moderate amount of cytoplasm devoid of granules (Fig 4). Promyelocytes with heavily granulated cytoplasm and bilobed nuclei, myelocytes and metamyelocytes were also seen (Fig 5). Based on the results of peripheral blood smear a diagnosis of acute myeloid leukemia was established. To subclassify AML, FAB classification was applied and the picture was suggestive of AML-M4 type.
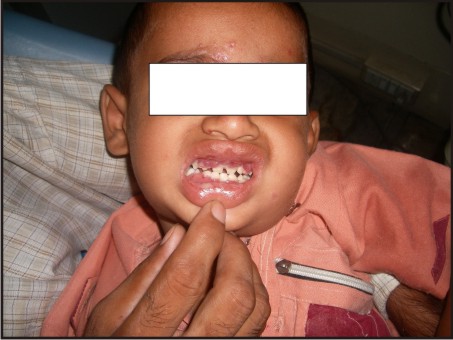 | Fig 1: Multiple Blisters On Forehead And Nose. Multiple Ulcers On Labial Mucosa
 |
 | Fig 2: Generalized Gingival Enlargement
 |
 | Fig 3: Peripheral Blood Smear Showing Multiple Blast Cells
 |
 | Fig 4: Blast Cells
 |
 | Fig 5: Premyelocyte And Myelocyte
 |
Discussion
Leukemia is the extremely fatal disease and 4% of death from cancer occurs due to this disease. Acute leukemia is the most common form of cancer in children. It can be further classified by determining whether the malignant leukocytes are of myeloid origin (granulocyte, monocyte, erythroid or megakaryocyte) or lymphoid origin (B-cells, T-cells). Acute myeloid leukemia (AML) is a relatively rare cancer, it accounts for 1.2% of all cancer deaths and 90% of all acute leukemias in adults[4]. It is seen less common in children as compared to acute lymphoblastic leukemia . The incidence increases with age, mean age being 63 years. It is slightly more common in men, with male to female ratio of 1.3:1[5].
The term “leukemia” was coined by Ruldolf Virchow in 1856. Wilhelm Ebstein introduced the term “acute leukemia” in 1889 to differentiate rapidly progressive and fatal leukemias from the more indolent chronic leukemias[6]. In 1900 the myeloblast, which is the malignant cell in AML, was characterized by Naegali, who divided the leukemias into myeloid and lymphocytic[5].
The malignant cell in AML is the myeloblast. In normal hematopoiesis, the myeloblast is an immature precursor of the myeloid white blood cells; a normal myeloblast will gradually mature into a mature white blood cell. However, in AML , a single myeloblast accumulates genetic changes which “freeze” the cell in its immature state and prevent differentiation[6]. Such mutation alone does not cause leukemia; however such a “differentiation arrest” is combined with other mutations which disrupt genes controlling proliferation, the result is the uncontrolled growth of an immature clone of cells, leading to the clinical entity of AML[7]. Specific cytogenetic abnormalities can be found in many patients with AML The chromosomal translocations encodes normal fusion proteins, usually transcription factors whose altered properties may cause the “differentiation arrest”[8]. In premyelocytic leukemia, the t(15;17) translocation produce a PML-RARα fusion proteins which binds to the retinoic acid receptor element in the promoters of several myeloid-specific genes and inhibits myeloid differentiation[9].
The symptoms of AML occur as a result of replacement of normal bone marrow with leukemic cells, which causes pancytopenia and increase in the immature cells[10]. Common symptoms include unexplained fever, weakness, fatigue, bleeding gums, skin bruising, cervical lymphadenopathy, gingival swellings, leukemic deposits, purpura and mucosal ulceration. Gingival swelling occurs due to the lack of normal healthy and mature leukocytes to fight the low grade gingival infections, as a result gingivae become packed with leukemic cells appearing swollen, purplish, necrotic and may ulcerate[10]. Enlarged spleen may also be noticed. All these symptoms may be due to bone marrow dysfunction leading to decreased normal white blood cells (causing infection & fever), decreased platelets (causing bleeding) and anemia (causing fatigue). Our case was a 3 year old male who presented with generalized gingival enlargement and multiple ulcers with intermittent episodes of fever and cervical lymphadenopathy. Splenomegaly was not present.
The first clue to a diagnosis of AML is an abnormal result on a complete blood count. While an excess of abnormal white blood cells (leukocytosis) is a common finding, and leukemic blasts are seen, AML can also present with isolated decrease in platelets, red blood cells, or even with a low white blood cell count (leukopenia). In the current case, peripheral blood smear stained by Romanowsky stain showed leukocytosis with increased blasts suggestive of myeloblasts. The blood picture was suggestive of Acute Myeloid Leukemia.
Based on the morphologic characteristics and cytochemical reactivity patterns of the predominate leukemic cell population the French-American-British (FAB) Corporation classified acute myelogenous leukemia into eight major subtypes(AML-M0 to AML-M7). Using this classification, the basic requisite for a diagnosis of AML is the presence of 30% or more blasts in the peripheral blood and/ or bone marrow[11].
Acute myelogenous leukemia (AML-M4) and (AML-M5) have the highest reported incidence of oral leukemic infiltrates[1]. The current case was diagnosed as AML-M4 subtype showing oral involvement in the form of mucosal ulcers and gingival enlargement .
Therefore, a presumptive diagnosis of AML can be made via examination of the peripheral blood smear when there are circulating leukemic blasts, and a definitive diagnosis requires an adequate bone marrow aspiration coupled with cytochemistry by myeloperoxidase, sudan black, peroxidase acid Schiff (PAS), non specific esterase (NSE) and acid phosphatase and immunophenotyping by markers CD33, CD13, CDw65, myeloperoxidase and/ or anti MPO[12],[13]. Treatment of AML consists primarily of chemotherapy with Cytarabine and an Anthracycline, and is divided into two phases: Induction or complete remission and Consolidation or postremission therapy. The goal of induction therapy is to achieve a complete remission by reducing the amount of leukemic cells to an undetectable level ; the goal of consolidation therapy is to eliminate any residual undetectable disease and achieve cure[14].
Conclusion
Acute myeloid leukemias are a heterogeneous group of diseases with diverse morphological, cytochemical, immunophenotypic and clinical characteristics with less prevalence amongst children. The treatment of AML is advancing rapidly and like therapy in other malignant states, is favouring treatment strategies tailored to specific leukemic subtypes. Though cytogenetic studies and immunophenotyping are more confirmatory, reproducibility of FAB classification on a peripheral blood smear should be carried out on a routine basis within the laboratories as it is easy to perform, does not require an elaborate setup, cheap, simple, better and less time consuming to identify.
References
1. Elisa Dorantes- Acosta et al. Acute myelogenous leukemia switch lineage upon relapse to acute lymphoblastic leukemia: a case report. Cases Journal 2009; 2:154.
2. Brad W. Neville, Douglas D .Damm, Carl M. Allen, Jerry E. Bouquot. Oral and maxillofacial pathology.3rd edition 2009; pg-587.
3. Jai Shree Sharma, Shobha Mohindroo. FAB classification of leukemia: a cytochemical study. Indian J Pathol Microbiol 2004; 47(3):336-339.
4. Jemal A, Thomas A, Murray T, Thun M. “Cancer statistics”. CA Cancer J Clin 2002 ; 52(1):23-47.
5. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. “Cancer statistics” CA Cancer J Clin 2001 ; 51(1):15-36.
6. Fialkow PJ. Clonal origin of human tumors. Biochem. Biophys. Acta 1976; 438(3): 283-321.
7. Fialkow PJ, Jansssen JW, Bartram CR. Clonal remissions in acute nonlymphocytic leukemia: evidence for a multistep pathogenesis of the malignancy. Blood Fia1991; 77(7):1415-7.
8. Greer JP et al. Wintrobe Clinical hematology l 11th edition . 2004.
9. Melnick A, Licht JD. Deconstructing a disease: RARα, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 1999; 93:3167-215.
10. Cawson RA, Odell EW. Cawson Essentials of oral Pathology and Medicine, 8th edition. 2008: pg-339.
11. Kay S. Amin, Aamir Ehsaan, H. Stan McGuff, Steven C. Albright. Oral Oncology 2002; 38:516-519.
12. Baldus CD, Mrozek K, Marcucci G, Bloofield CD. Clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics is affected by molecular genetic alterations. Br J Haematol. 2007; 137:387-400.
13. Sharmila Ghosh, et al. Acute myeloid leukemia presenting with an unusual phenotype. Indian J Med Paedia Onco 2005; 26(1):53-57.
14. Bishop J. The treatment of adult acute myeloid leukemia. Semin Oncol 1997; 24(1): 57-69.
|