Introduction
The success of endodontic therapy depends on complete periapical repair and regeneration. Most of the time teeth with periapical lesions heal satisfactorily after nonR09;surgical endodontic intervention. Abramovitz et al. discussed the guidelines of case selection for apical surgery and nonR09;surgical retreatment. They reported that treatment of 24.5% of the cases was impossible without surgical therapy[1]
The healing of hard and soft tissue is mediated by wide range of intra and extracellular events that are regulated by signaling proteins. Platelets play a crucial role not only in hemostasis, but also in wound healing process.
Platelets are formed in bone marrow from megakaryocytes. Their lifespan is 8 to 10 days, and their cytoplasm contains many granules whose contents are secreted at the time of activation.
αR09;Granules contain many proteins, platelet specific (such as βR09;thromboglobulin) or nonplatelet specific (fibronectin, thrombospondin, fibrinogen, and other factors of coagulation, growth promoters, fibrinolysis inhibitors, immunoglobulins, etc.). The dense granules contain calcium, serotonin etc.
Activation is fundamental to initiate and support hemostasis because of aggregation of platelets on the injured site and interactions with coagulation mechanisms. However, degranulation implies the release of cytokines and ability to stimulate cell migration and proliferation within the fibrin matrix, launching the first stage of healing[2].
Case Report
A 27 year old male patient came to the Department of Conservative Dentistry and Endodontics, Genesis Institute of Dental Sciences and Research with chief complaint of pus draining from upper front region. He had a history of trauma 4 years back. On clinical examination 21, 22 were slightly tender on percussion and pulp vitality tests were negative. On radiographic examination, there was a presence of a large peri-apical radiolucency with respect to 21, 22 and the defect was starting from middle third of the root and extend to the apical area [Figure 1]. Diagnosis of Chronic Irreversible Pulpitis with Periapical abscess was made. After analyzing the case radiographically, clinically and, it was decided to proceed with the surgical approach. The patient was explained in detail about surgical treatment planning and the regenerative modality to be used.
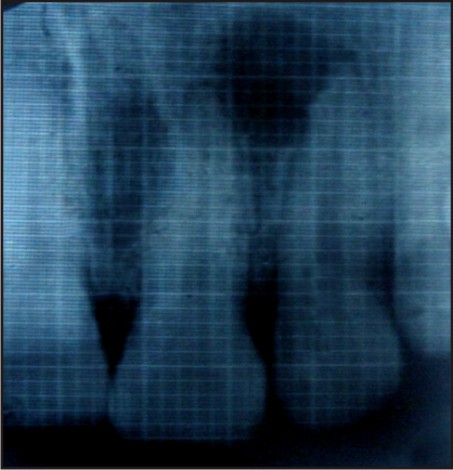 | Figure 1: Peri-apical Radiolucency With Respect To 21, 22
 |
Multipe visit endodontic treatment was performed with calcium hydroxide placed as an intracanal medicament for 3 weeks. Hyposol (3% sodium hypochlorite)was used to irrigate the canals during the canal preparation. The root canals were obturated by the lateral compaction technique.
Surgical Technique:
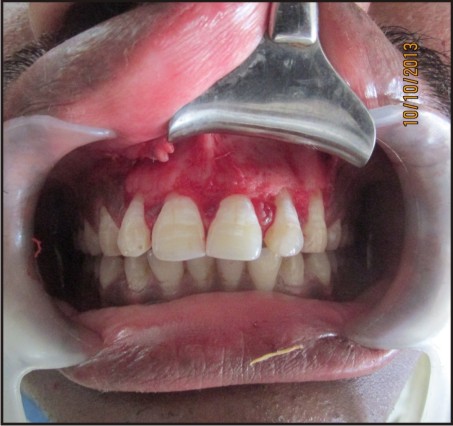
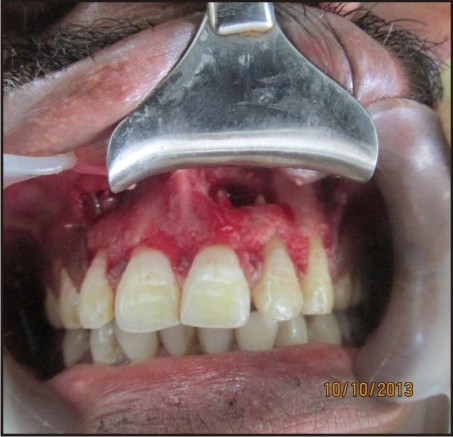
Under local anesthesia, lignocaine 2% with 1:200000 adrenaline (LOX 2% adrenaline), a full thickness muco-periosteal flap was reflected by a sulcular incision starting from the distal of the tooth #12 to distal of the tooth #23 [Figure 1(a, b)]. Window preparation was done with respect to #21 and #22. [Figure 1(c)]. Tissue curettage was done at the defect site followed by thorough irrigation using sterile saline solution. Using #702 tapered fissure bur (SS White burs), root end resection was performed wrt #21 and #22[Figure 1(d)] and white mineral trioxide aggregate (MTA Angelus, Londrina, PR, Brazil) was used as the root end filling material and platelet rich fibrin (PRF) was placed in the bony cavity.
 | Figure 1(a): Intra-oral View
 |
 | Figure 1(b): Incision Given And Full Thickness Muco-periosteal Flap Reflected
 |
 | Figure 1(c): Window Preparation And Apical Thirds Of Root Exposed
 |
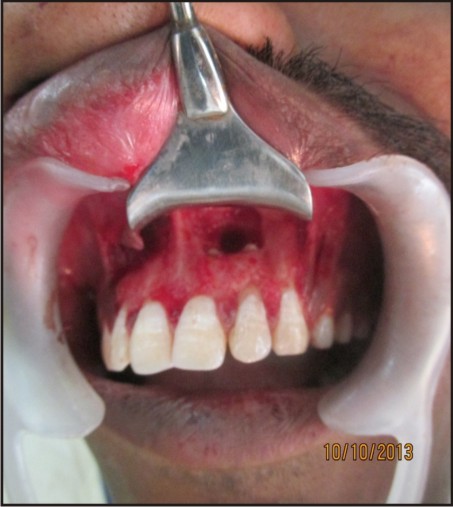 | Figure 1(d): Root Resection Done
 |
Protocol for PRF preparation
The protocol for PRF preparation is very simple. 10 ml of venous blood was drawn from the patient. Whole blood was drawn into vacutainer tubes without anticoagulant and immediately centrifuged at 3,000 rpm for 10 minutes.
Within a few minutes, the absence of anticoagulant allows activation of the majority of platelets contained in the sample to trigger a coagulation cascade. The result is a fibrin clot containing the platelets located in the middle of the tube, just between the red blood cell layer at the bottom and acellular plasma at the top.
This clot was removed from the tube and the attached red blood cells scraped off and discarded [Figure 2]. PRF gel was carefully placed into the cavity till the entire cavity was filled [Figure 3(a,b)].
Wound closure was performed with a 3R09;0 black silk suture [Figure - 4].
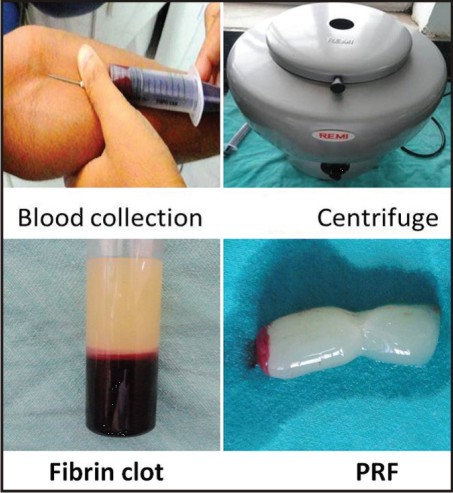 | Figure 2: Prf Preparation
 |
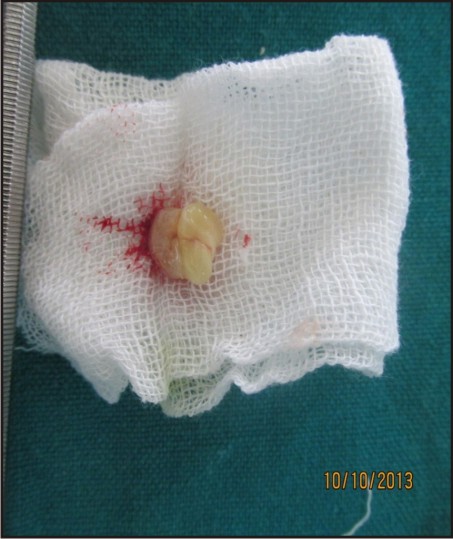 | Figure 3(a):Prf Obtained From Patient’s Blood
 |
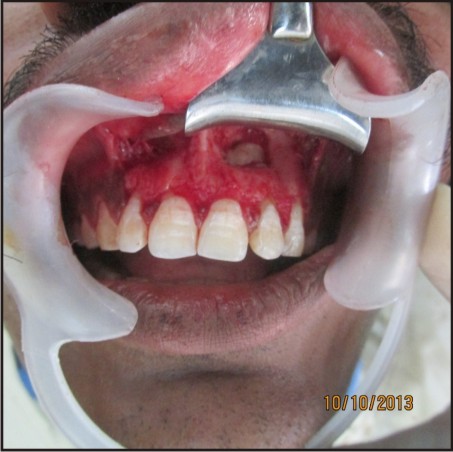 | Figure 3(b):Prf Placed In Bony Cavity
 |
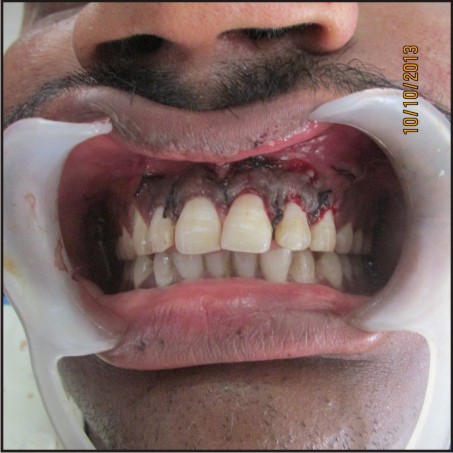 | Figure 4:Placement Of Sutures
 |
Post-operative antibiotics, Augmentin (combination of Amoxicillin and Clavulanate Potassium) 625mg tablet every 8 hourly for 5 days, Metrogyl (Metronidazole) 400mg twice a day for 3 days and anti-inflammatory i.e., Enzoflam (combination of Diclofenac, Paracetmol and Serratio-peptidase) 8 hourly for 3 days were prescribed. The sutures were removed after seven days. The patient was reviewed after 3, 6 and 9 months [Figure 5, 6, 7] during which there were no symptoms of pain, inflammation, or discomfort. These follow-up visits included routine intraoral examinations and professional plaque control.
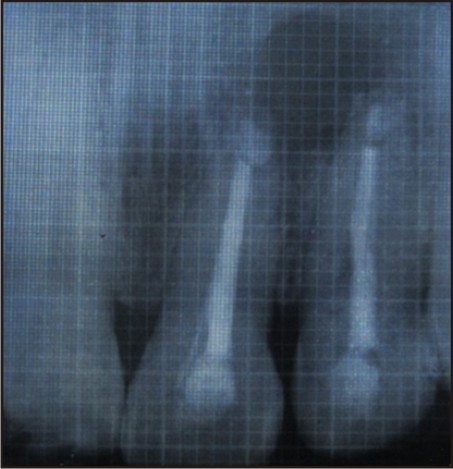 | 3 Months Follow Up
 |
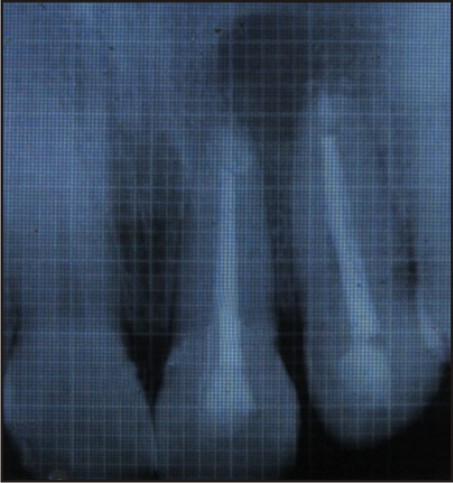 | 6 Months Follow Up
 |
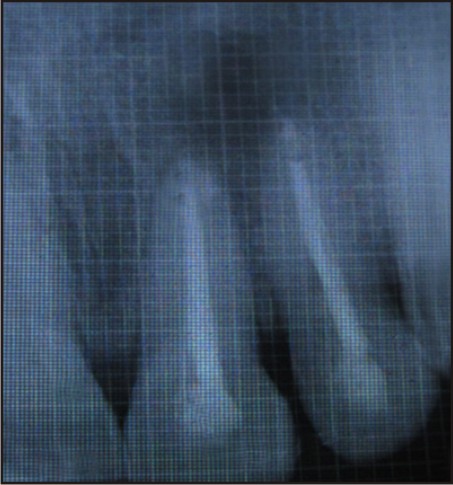 | 9 Months Follow Up
 |
Results
The patient did not complain of any unusual or severe pain. There were no signs of infection, untoward reaction or wound dehiscence. Radiographically, patient showed satisfactory regeneration at the end of 9 months.
Discussion
The understanding of how normal wound healing and tissue formation occurs together with recent advances in materials science, stem cell research and developmental biology have helped to find target molecules and pathways, which can restore a patient’s regenerative capacity[3]
Regeneration of tissue after periapical surgery requires (a) recruitment of progenitor/stem cells to differentiate into committed cells, (b) growth / differentiation factors as necessary signals for attachment, migration, proliferation and differentiation of cells, and (c) local micro-environmental cues like adhesion molecules, extra cellular matrix, associated non-collagenous protein molecules, and so forth[4].
To promote periapical tissue regeneration and healing, local application of growth factors and host modulating agents is being used to maximize the body’s healing potential. TGF-beta and PDGF are the typical two growth factors which promote healing of soft tissue and bone through stimulation of collagen production to improve wound strength and initiation of callus formation[5]. PDGF is a regulator formigration, proliferation, and survival of mesenchymal cell lineages. TGF-beta constitutes the most powerful fibrosing agent among all cytokines. It induces massive synthesis of matrix molecules such as collagen-I and fibronectin either by osteoblasts or fibroblasts. Although its regulation mechanism is particularly complex, it is considered as an inflammation regulator through its capacity to induce fibrous cicatrization. Basic studies have demonstrated that specialized secretory granules of platelets, such as alpha-granules, contain these growth factors[6]. Growth factors are known to attract stem cells present in apical tissues[7] This property of PRF finds application in healing of large osseous defects where there is migration of stem cells differentiating into osteoblast phenotype[8]
In vitro studies have proved that PRF releases autologous growth factors gradually for at least 1 week and up to 28 days [9]. The natural and slow polymerization occurring during centrifugation process of PRF leads to formation of a homogenous 3-dimensional organization of the fibrin network. The absence of anticoagulant in the test tube leads to massive platelet activation, bolstered by the presence of a mineral phase on the tube walls (residual glass particles). A progressive polymerization mode signifies increased incorporation of the circulating cytokines in the fibrin meshes (intrinsic cytokines). This configuration increases the lifespan of these cytokines, as they are released and used only at the time of initial cicatricial remodeling . PRF has a stronger and more durable effect than PRP[10]
MTA was used as a root-end filling material because of its advantages such as biocompatibility, antibacterial action, less micro leakage, good sealing ability, alkaline pH (Torabinejad et al. 1995b, 1997); the presence of calcium and phosphate ions in its formulation (Torabinejad et al. 1995b); the capacity to attract blastic cells and to promote a favourable environment for cementum formation (Pitt Ford et al. 1995, Torabinejad et al. 1995a); osseous and cementum-conductive effect (Shabahang et al. 1999, Moretton et al. 2000); the stimulus to adhesion and cell proliferation (Koh et al. 1998), stimulus to expression of alkaline phosphatase by fibroblasts (Bonson et al. 2004) and osteocalcin and other interleukins by osteoblasts (Koh et al. 1997, Mitchell et al. 1999). When used as retrograde root filling material, MTA also demonstrated high success rate when compared to other materials such as zinc oxide eugenol cement, glass-ionomer cement, Super ethoxy benzoic acid, Composite[11].
In this case, the radiograph reveals satisfactory bone regeneration after 9 months. Thus, it can be considered that PRF is a healing biomaterial, as it features all the necessary parameters permitting optimal wound healing.
Conclusion
Early publications and clinical experience seem to indicate that PRF improves early wound closure, maturation of bone, and the final aesthetic result of the periodontal soft tissues.
Production of a dense, crossR09;linked, physically robust PRF made of intact platelets and fibrin by highR09;speed centrifugation in the absence of exogenous thrombin, yields an ideal scaffold for use in tissue repair. The conversion of fibrinogen into fibrin in PRF takes place slowly with small quantities of physiologically available thrombin present in the blood sample itself. Thus, a physiologic architecture that is very favorable to the healing process is obtained due to this slow polymerization process.
References
1. I Abramovitz et al. Case Selection for apical surgery: A retrospective evaluation of associated factors and rational. Journal of Endodontics 2002; 28:527R09;30.
2. J Choukroun et al. PlateletR09;rich fibrin (PRF): A secondR09;generation platelet concentrate. Journal of Oral Surgery Oral Medicine Oral Pathology Oral Radiology Endodontology, 2006; 101:45R09;50.
3. Chen FM et al. A review on endogenous regenerative technology in periodontal regenerative medicine. Journal of Biomaterials 2010; 31:7892-927.
4. L. Lin et al: Guided tissue regeneration in periapical surgery, Journal of Endodontics, 2010, 36(4), 618–625.
5. JD. Bashutski and H. L. Wang: Periodontal and endodontic regeneration, Journal of Endodontics, 2009; 35(3), 321– 328.
6. XE Dereka, CE Markopoulou, and IA Vrotsos: Role of growth factors on periodontal repair, Growth Factors, 2006; 24(4), 260–267.
7. M. Torabinejad and M. Turman: Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. Journal of Endodontics, 2011; 37(2), 265–268.
8. Choukroun J, Diss A, Simonpieri A et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate, part IV: Clinical effects on tissue healing. Journal of Oral Surgery Oral Medicine Oral Pathology Oral Radiology Endodontolgy; 2006;101:E56–60
9. L He, Y Lin, X Hu, Y Zhang, and H Wu. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Journal of Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology; 2009,108(5), 707–713.
10. J Choukroun et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Journal of Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology, 2006, 101(3), E45–E50.
11. HW Roberts, JM Toth, DW Berzins, DG Charlton. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Journal of Dental Materials; 2008;24:149-64.
|