Introduction:
In earlier times when living was simple so were the means of committing crime. With technical advancement and easy accessibility to everything, the nature of both living and killing has changed by leaps and bounds. From unimaginable feats of human grandeur to unwanted lows of grisly human crimes, humans have come a long way. In earlier times criminals employed very easy to use and technically not sound weapons for killing. But now a days with more knowledge even criminals have become highly advanced using every mean to escape conviction. For this reason rather than previous means of killing and then dumping a body, they are using materials like acids to completely destroy the body hence any identification. [1]
For such crimes the forensic expert needs to be well versed with the changes caused by these acids on body tissues to correctly identify the deceased. Natural teeth are one of the most durable organs. Teeth and dental restorative materials are generally impervious to decay or destruction by physical agents like acids and fire, though both of them can alter them considerably. Hence the role of forensic dentists becomes crucial in crimes involving these agents. Search through medical literature has revealed very few studies demonstrating the effect of acids on morphology of dental tissues.[1],[2]
Criminals are always guided in their choice by ease of availability of the acid, cost and efficiency of action. They will be more likely to use an acidic agent that is easily available, cheap and with ability to destroy the body rapidly. The commonly available and less expensive acids in the market are hydrochloric acid and sulfuric acid whereas nitric acid is little steep in its price.[1],[3] The studies evaluating the effect of acids on teeth are pertaining to use of strong acids like hydrochloric acid, sulfuric acid and nitric acid mainly. Other acids are very rarely used for eg. hypochlorous acid, which is formed when chlorine compounds dissolve in water. Chlorine is normally used for disinfection in swimming pools but in excess can lead to formation of acidic radicals which are destructive to human tissues immersed in it. [4],[5]
The aim of this pilot study was to identify the morphological and radiological changes of natural human teeth in an acid environment and to collate the reference data in order to assess the applicability of such changes for identification of acid used and time elapsed.
Materials And Method:
Forty extracted permanent anterior teeth were used for the study which was conducted in the department of Oral Pathology and Microbiology of Sudha Rustagi College of Dental Sciences and Research, Faridabad. All these teeth were non carious and were extracted for periodontal reasons. Teeth were thoroughly cleaned with distilled water and kept at room temperature.
The acids used for the study were:
1. 50 ml of 37% Conc Hydorchloric Acid (HCl) (Merck Specialities Pvt Ltd, India).
2. 50 ml of 98% Conc. Sulphuric acid (H2SO4) (Merck Specialities Pvt Ltd, India).
3. 50 ml of 70% Conc. Nitric acid (HNO3) (Merck Specialities Pvt Ltd, India).
4. 50 ml of 100% Hypochlorous acid (HOCl) (Merck Specialities Pvt Ltd, India).
Ten teeth each were kept in each of the four solutions in clean glass beakers at room temperature. For the periodic assessments the teeth were washed in distilled water, dried, photographed, radiographed and again placed in their respective acids. Time intervals along with morphological and radiological assessment data are mentioned in Table 1. The specimens were kept under observation till they completey dissolved or precipitated by action of acids.
Results:
The results have been tabulated in the following Table 1:
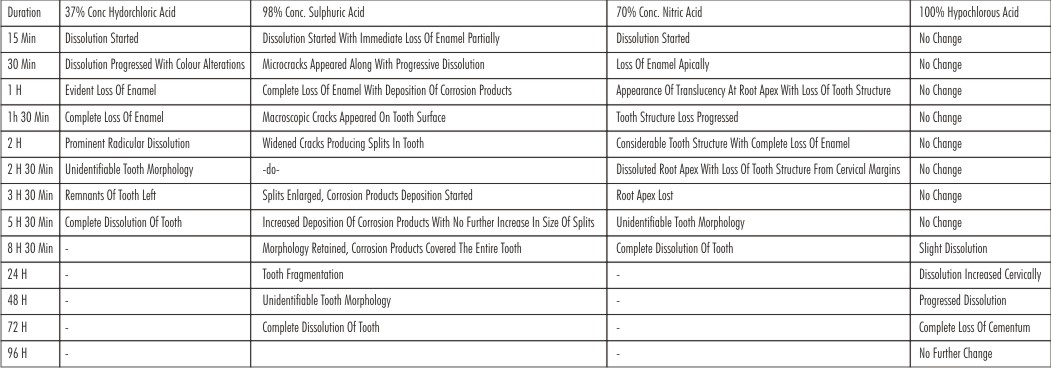 | Table 1. Clinical And Radiological Assessment Data
 |
Discussion:
The face of crime is changing with advancing era and the modus operandi is becoming more appalling. Earlier weapons of crime are being replaced by newer weapons like acids to completely destroy the body and to escape from leaving any evidence behind. The identification of such destroyed body becomes a challenge for forensic experts. It is well known that the forensic odontological techniques are suitable to aid the identification process because natural teeth are one of the most durable structures in human body and impervious to destruction generally. Natural human teeth can persist long after other skeletal structure had decayed and/or destroyed by physical and chemical agents like incineration and acids respectively. Thus, our study was undertaken to identify the morphological changes induced in teeth if immersed in various commonly used acids and to assess the applicability of such changes for identification of acid used and time elapsed since death.[1], [2], [3]
Intact non carious teeth were used for this study as it has been found that presence of carious lesion in a tooth can weaken its structures thus enabling easy penetrability of acids hence accelerating the acid attack with profound effects.
An acid is a substance that produces hydronium ions (H3O+) when it is added to water by donation of an H+ ion, and thus an acidic solution is one which contains significant concentration of H3O+ ions. A strong acid, such as hydrochloric acid (HCl)(aq), nitric acid (HNO3) and sulfuric acid (H2SO4) are substances that undergoes a completion reaction with water so that each acid particle reacts to form a hydronium ion (H3O+). Thus strong acids form nearly one H3O+ ion in solution for each acid molecule dissolved in water, making them more acidic hence more harsh or caustic. Whereas a weak acid like hypochlorous acid (HOCl) is a substance that is incompletely ionized in water because of the reversibility of its reaction with water that forms hydronium ion (H3O+). [6], [7], [8], [9]
Hydrochloric, nitric and sulfuric acid are industrial and laboratory grade acids commonly available.[7],[8],[9] Whereas, HOCl is commonly formed when chlorine compounds are added to swimming pools for killing bacteria and viruses. The active chemical is HOCl (hypochlorous acid), which passes through the cell wall and oxidizes or “burns up” the interior of the bacterium.[5] In some crimes bodies have been found to be dumped in far flung located and unused swimming pools with dirty water. Search through medical review hasn’t revealed any substantial study pertaining to effect of Hypochlorous acid on dental tissues.[10] Hence the uniqueness of this study was the use of this acid.
These chemicals and their reactions with the body’s water content, validate the use of both strong and weak acids in present study apart from other factors like ease of their availability and cost factor. A criminal mindset favors the use of acids which are easy to obtain, fast acting, inexpensive and more deleterious.
It has been found that sulfuric acid and hydrochloric acid are commercial grade acids easily available in the concentrations used in the study unlike nitric acid which is costlier and relatively difficult to obtain. Hypochlorous acid usage requires more studies since data pertaining to its use in such crimes is very limited.[1],[3]
Dental erosions caused by acids starts reacting with enamel first followed by dentin. Reaction of acids with crystallites of hydroxyappatite [Ca10(PO4)6OH2] in enamel leads to dissolution or demineralization of enamel surface as shown in equation below:
Ca10(PO4)6OH2(s) + 8H+(aq) à 10Ca2+(aq) + 6HPO42-(aq) + 2H2O
These free calcium ions further react differently with different acids leading to the formation of soluble and insoluble calcium salts. [3],[11]
Teeth placed in 37% conc.HCl dissolved completely in 5 hours and 30 mins. The chemical process lead to the formation of calcium chloride salts which is completely soluble in water. Radiographically, there was gradual enamel loss and prominent radicular portion loss eventually leading to unidentifiable tooth morphology.
Teeth + HCl à Calcium chloride (soluble)
Teeth placed in 70% conc. HNO3 dissolved completely in 8 hours and 30 mins. The chemical process lead to the formation of calcium nitrate salts which is completely soluble in water. Radiographically, initially enamel was lost followed by increased cervical tooth structure loss, root loss and ultimately ample tooth structure loss.
Teeth + HNO3 à Calcium nitrate (soluble)
Since the salts formed by above two acids were fully soluble hence the teeth got completely dissolute in these acid solutions leaving behind no residues. On the other hand, teeth placed in 98% conc. H2SO4 demonstrated their gross dissolution in 72 hours, leaving residual components at the end of the observation period. There was progressive fragmentation in form of cracks in teeth which were apparent both macroscopically and radiographically, instead of dissolution. Fragmentation could be due to chemical reaction not acting as a homogeneous procedure, but in the formation of fissures in a similar way as is found in crevice corrosion process typically seen in metals. The chemical process lead to the formation of calcium sulfate salts which were insoluble in water. [1]
Teeth + H2SO4 à Calcium sulfate (insoluble)
Immersion of teeth in 100% HOCl lead to formation of insoluble calcium hypochloride salts, without complete destruction even in 96 hrs. Radiographically, there was increased tooth dissolution cervically with loss of enamel and cementum.
Teeth + HOCl à Calcium hypochloride (insoluble)
These insoluble salts got deposited on tooth surface altering the morphology of the teeth. Based on these results it can be deduced that different acids act variably in destructing the tooth surface either by causing complete tooth dissolution or by forming insoluble deposits.
Conclusion:
On the basis of the results, it is possible to observe differences in the destructive capacity of the acids used. The complete destruction of teeth without residues was seen as a progressive phenomenon in case of 37% conc. HCl and 70% conc. HNO3 which were assessed radiographically as well, demonstrated by the complete loss of tooth structure. On the other hand 98% conc H2SO4 showed partial destruction of teeth leaving behind residual components. Radiographically there was loss of enamel, appearance of eventually widening cracks with loss of tooth morphology. Whereas teeth immersed in 100% conc. HOCl showed only partial destruction at the end of observation period. Radiographical analysis showed vey mild changes with HOCl.
With this information and by referring to this data possibility of deduction of time elapsed and the type of acid used exist.
Although our experiment did not take into account possible factors present in real life conditions i.e. the protection provided by soft and hard tissues surrounding the dental components or devices and the effect of dentures and other prosthesis on acid dissolution. These tissues and materials prevent direct exposures to acids.
When it is no longer possible to identify dental structures that have been dissolved in acid, other types of investigations are available: a biochemical or histological analysis of the residues, the possibility of an eventual DNA analysis, and the chemical analysis of final solutions and residues.
References:
1. Mazza A, Merlati G, Savio C, Fassina G, Menghin P,Danesino P. Observations on Dental Structures when Placed in Contact with Acids: Experimental Studies to Aid Identification Processes. J Forensic Sci, Mar. 2005, Vol. 50, No. 2:1-5.
2. Carr R.F, Barsley R.E, Davenport W.D. Postmortem examination of incinerated teeth with the scanning electron microscope. Journal of Forensic sciences, JFSCA 1986,vol 31,no 1: 307-311.
3. Jadhav K, Gupta N, Mujib BRA, Amberkar VS. Effect of acids on the teeth and its relevance in post mortem identification. J of Forensic Dental sciences 2009; 1(2): 93-8.
4. http:// www.dh.sa.gov.au / pehs / publications / code-swimpool-oper.pdf
5. http://portal.acs.org / portal / fileFetch / C / WPCP_010102 / pdf / WPCP_010102.pdf
6. http://preparatorychemistry.com / bishop_study_guide_atoms_8.pdf
7. http://www.cdc.gov/niosh/docs/81-123/pdfs/0447.pdf
8. http://www.hpa.org.uk / webc / HPAwebFile / HPAweb_C / 1194947355794
9. http://www.jsia.gr.jp / data / handling_02e.pdf
10. http://boston.cbslocal.com / 2011/06/30/state-closes-all-30-deep-water-pools-after-body-goes-unnoticed-for-days/
11. http://webcat.warwick.ac.uk:80 / record=b2581400~S1
|