Introduction
Advances in Endodontic therapy have enabled the clinician to retain teeth previously destined for extraction. Over the years, the procedure of RCT has been refined and perfected with a more predictable outcome. Bacteria and their products play an essential role in the initiation and perpetuation of pulpal and periapical diseases.[1] Theoretically, all bacteria present in the oral cavity may invade the root canal during or after pulp pathosis, and thereby participate in Endodontic infections. However, because of bacterial interactions and varying oxygen pressures inside the root canal, bacteria present in Endodontic infections include a restricted group of species.[2] The predominant organisms are obligate anaerobes, mainly gram negative such as black pigmented rods and fusobacteria.[2]
Researchers recovered a number of species of anaerobic bacteria from failed root canal treated systems. Some of these bacteria include Enterococcus faecalis, Streptococcus sanguis, Bacteroides gracillis and Fusobacterium nucleatum. From all these cases studied, Enterococcus faecalis was found to be the most prevalent bacteria in failed root canal treated systems. Therefore, the elimination or reduction of bacteria from the root canal[3] forms one of the components of the triad in root canal therapy. Mineral Trioxide Aggregate (MTA) was introduced to dentistry by Torabinejad et al in 1993 and has been used successfully as a root- end filling material. It has been advocated for repairing perforations, pulp capping, and apexification.[4] MTA reacts with tissue fluids to form an apical barrier. As a result, MTA shows promise as a valuable material for use in one-visit apexification treatment, primarily for treating immature teeth with necrotic pulps.[4] Chlorhexidine is a broad spectrum antimicrobial agent[5] and at low concentrations it is bacteriostatic[6] and at higher concentrations Chlorhexidine will cause the coagulation and precipitation of cytoplasm and therefore is bactericidal.
Most of the endodontic failures are attributable to inadequate cleansing of the root canal and egress of bacteria and other antigens into periradicular tissues. Therefore, in addition to having good sealing ability and biocompatibility root end filling material should ideally have some anti microbial activity to prevent bacterial growth.[7]
The present study is to observe antimicrobial efficacy of MTA with Chlorhexidine, as this aspect will form a sound scientific foundation for the effective use or the acceptability of the combination in clinical situation.
The objective of this ex-vivo study is to evaluate the antimicrobial efficiency of MTA with 0.12% chlorhexidine gluconate and sterile water on:
1. Enterococcus faecalis
2. Pseudomonas aeruginosa
3. Candida albicans.
4. Escherichia coli
5. Staphylococcus aureus
The aim of this ex vivo, agar-diffusion study is to determine whether substitution of Chlorhexidine Gluconate in place of sterile water as a mixing agent would enhance this antimicrobial activity.
Materials & Method
The present ex-vivo study has been undertaken in the Post-Graduate Department of Conservative Dentistry and Endodontics, S.P.P.G.I.D.M.S., Lucknow in association with the Department of Microbiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow.
Armamentarium
1. Chlorhexidine (0.12%)
2. Sterile water
3. MTA-Mineral Trioxide Aggregate (Proroot, Dentsply Mallifer),
4. Test tubes (3 x 4 cm) - 56
5. Test tube holders
6. Ultrasonic stirrer
7. Agar plates
8. Antibiotic zone scale
9. Incubator
10. Luria Bertani broth
11. Brain heart infusion broth
12. Sabouraud’s dextrose media
13. Spirit lamp
14. Inoculating tubes
15. Drier
16. Micropippetes (20 µl, 200µl, 1000µl)
17. Cotton swab with wooden stick
The selected microorganisms were:
a) Standard Strains of Candida albicans ATCC 90028
b) Standard Strains of Pseudomonas aeruginosa ATCC 27853
c) Standard Strains of Staphylococcus aureus ATCC 25923
d) Standard Strains of E. coli ATCC 25922
e) Standard Strains of Enterococcus faecalis ATCC 29212
Experimental groups were:
A) Mineral Trioxide Aggregate with Chlorhexidine Gluconate
B) Mineral Trioxide Aggregate with sterile water
C) Chlorhexidine
D) Sterile water
Method
ATCC strains of Enterococcus faecalis, Escherichia coli and Pseudomonas aeruginosa were inoculated into 5 ml samples of Luria Bertani broth and incubated aerobically for 24 hrs at 37° C.
Standard strains of Staphylococcus aureus was grown in brain heart infusion broth and incubated aerobically for 24 hrs at 37° C.
ATCC strains of Candida albicans was grown in Sabouraud’s dextrose media at 37° C and incubated aerobically for 24 hrs.
One hundred - micro liter aliquots of each suspension (standardized to McFarland 4) were spread on 25 agar plates. Five plates per strain for the test. After inoculation, two wells of 5 mm diameter were made in the agar.
One pack of (100mg) of MTA is mixed with 36 micro liters of 0.12% CHG or sterile water.
The resultant mixture is divided into three equal parts; one part is transferred to a well on each plate so that each of the 25 experimental agar plates had one well filled with the MTA / CHG mixture (33mg MTA/12microlitre Chlorhexidine per well) and the other filled with the MTA / Water mixture (33mg MTA/12microlitre sterile water per well). Fives independent assays were performed for each of the microorganisms.
A control Mueller Hinton agar plate for each of the microorganisms was made using sterile paper disc of 5 mm diameter from Whatman filter paper placed on opposite sides of the agar plate saturated with equal 12-µl samples of 0.12% CHG or sterile water were used to saturate each disc.
All plates were incubated at 37°C for 24 hrs and the zones of diameters were measured after 24 hours.
Criteria For Evaluation
The antimicrobial sensitivity pattern represented as the zone of inhibition was measured around each disc. Antibiotic zone scale was used for this purpose and measurements of each zone were recorded in millimeters.
Results And Observations
• Water did not show any antimicrobial activity at all. However, Chlorhexidine showed maximum antimicrobial activity against C. albicans (18 mm) while minimum for E. faecalis (10 mm) (Table: 1)
• MTA was always inhibitory regardless of the mixing liquid used
• The mean antimicrobial efficacy of Chlorhexidine with MTA was significantly higher as comparedto Water+MTA. (Table:2)
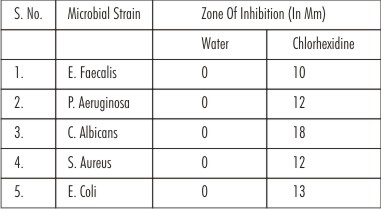 | Table 1 : Zones Of Inhibition Seen For Sterile Water And Chlorhexidine
 |
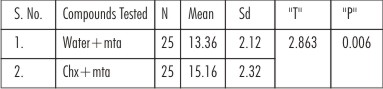 | Table 2 : Antimicrobial Efficacy Of Mta With Sterile Water And Mta With Chlorhexidine (Overall Antimicrobial Efficacy)
 |
Discussion
MTA has been used successfully in the repair of lateral root perforations, furcal perforations, as a vital pulp capping agent, as an apical plug in one visit apexification cases. As root-end filling material, MTA has the ability to stimulate osteoblast activity.[4] Since 2002, MTA has been marketed as white colored ProRoot Mineral Trioxide Aggregate.
According to Torabinejad et al.[8] MTA was divided into calcium oxide and calcium phosphate. Further analysis demonstrated that the former appeared as discrete crystals and the latter as an amorphous structure with no apparent crystal growth but a granular appearance. Since MTA[9] can be compared to Portland cement, the water confined in hardened Portland cement finds itself within a structure saturated with calcium hydroxide. This calcium hydroxide is mainly the soluble fraction of set Portland cement. When the mix comes in contact with an aqueous environment of a different saline concentration, Portland cement absorbs water. The water trapped in the cement mix and saturated by calcium hydroxide is then released to the environment. The pores and capillaries of the structure, now empty absorb fresh water. This water is again released along with the calcium hydroxide that has dissolved. In this way, water is continuously diffusing in and going out of the mass. In the case of Mineral Trioxide Aggregate once placed in situ and left undisturbed there are very less chances of influx or efflux of fluid process in the cement mass because the surrounding tissues are already saturated with fluid. This situation will end up in making set MTA a structure containing capillaries filled with fluid rich in calcium hydroxide. This justifies the prolonged and sustained antimicrobial effect of MTA. Thus, MTA possesses antimicrobial activity because of its high Ph 12.5 and several studies have investigated this activity (Khalid Al-Hezaimi et al., 2005[10]; Ayce Unverdi Eldeniz et al. 2006[11]; Khalid Al-Hezaimi et al., 2006[12]).
Chlorhexidine has been suggested as an irrigation solution and an intracanal medication. Chlorhexidine is a cationic molecule consisting of two 4-chlorophenyl rings and two bisbiguanide group with optimal antimicrobial action over the pH range of 5.5 to 7.0.[13] Chlorhexidine has been proven to have strong antibacterial activity against a wide range of microorganisms (Gram positive, Gram negative, Yeast, fungi, Facultative anaerobes and aerobes.[13]
In this study, microorganisms were selected to represent aerobes, anaerobes and yeast found in infected root canals. In particular, E. faecalis was chosen because it is the most frequently isolated microorganism recovered from failed endodontically treated cases.[3] C. albicans has also been demonstrated in root filled teeth with chronic apical periodontitis.[14]
In the present study, the results showed that MTA/CHG mixtures produced greater zones of inhibition than the MTA/Water mixtures. Results of this study also revealed that zone of inhibition for Enterococcus faecalis with MTA/CHG is 14.00 mm, whereas MTA/water is 11.40mm. As a consequence of high pH of MTA[11] and due to the presence of Chlorhexidine with MTA an enhanced antimicrobial action is produced against Enterococcus faecalis. The results of present study are in accordance with those of Sipert et al.[11] and Khalid Al Hezaimi et al.[12] who reported that MTA either delayed or inhibited the growth of Enterococcus faecalis.
Staphylococcus aureusare sometimes recovered from root canals. It represents a standard organism in antimicrobial testing. Mean zone of inhibition with MTA/CHG is 16.20 mm whereas zone of inhibition with MTA/water is 13.0mm.
It was seen that MTA/CHG mixtures produced greater zones of inhibition than the MTA/Water mixtures.
Torabinejad el al. (1995)[7] reported that Loma Linda MTA did not have an inhibitory effect against Enterococcus faecalis and Staphylococcus aureus. In the present study, the MTA/ Water mixture was inhibitory to Staphylococcus aureus, Enterococcus faecalis. This could be because of one of several differences between the studies. First, placement of the materials differed. In the previous study by Torabinejad el al. (1995)[7] experimental materials were placed directly on the surface of the agar before incubation but in the present study, wells were created in the agar into which the test materials were condensed. Another reason could be the different formulations of MTA. In the earlier study the Loma Linda MTA used might be different from the commercially marketed ProRoot Mineral Trioxide Aggregate.
Goran Sundqvist et al.(1994)[2] quoted that aerobic bacteria are very rarely found initially in infected root canals but may be introduced into the canal during the treatment. Pseudomonas aeruginosa is an example of such an organism. Results of present study revealed that mean zone of inhibition for Pseudomonas aeruginosa with MTA/CHG is 12.20 mm whereas MTA/water mixture revealed mean zone of inhibition to be 11.60 mm. MTA/water mixture seemed to have an equal inhibitory effect with the MTA/CHG mixture on Pseudomonas aeruginosa. Although this occurred in two of the five assays, additional studies may clarify this anomaly. Because controls of sterile water showed no inhibition and controls of Chlorhexidine always were inhibitory, it is concluded that the Chlorhexidine in the MTA mixtures was the component producing the enhanced antimicrobial response. The antibacterial effect of proroot Mineral Trioxide Aggregate against P. aeruginosa is likely explained by its high pH.[11]
Candida albicanshas the ability to form biofilms on different surfaces.[10] Biofilm helps in the adherence of the microorganism onto the canal wall. This property is one of the reasons why this species is considered to be more pathogenic than species that are less able to form biofilm.[10] Chlorhexidine helps to remove this biofilm and produces an enhanced antimicrobial response. Ferguson et al found that an aqueous solution of calcium hydroxide had no activity against Candida albicans. However when maintained in direct contact with Candida albicans cells, calcium hydroxide paste was very effective in killing this fungus. Therefore, it can be assumed that MTA is more effective in killing Candida albicans when placed in direct contact with the fungus.[15] Results of this present study revealed that Maximum efficacy is seen against Candida albicans 18.20 mm as shown by MTA/ CHG whereas zone of inhibition with MTA/ water is 16.60 mm. These findings agree with those of Khalid Al Hezaimi et al.[16] who reported that MTA may exert an antifungal action against Candida albixcans for periods of up to 3 days.
Escherichia coli are also commonly isolated from infected root canals and the mean zone of inhibition with MTA/Chlorhexidine is 15.20 mm whereas zone of inhibition with MTA/water is 14.20 mm. It was seen that MTA/Chlorhexidine mixtures produced greater zones of inhibition than the MTA/Water mixtures.
According to Mahmut Summer et al.[17] MTA mixed with Chlorhexidine materials created weak inflammatory responses characterized by the presence of fibrous connective tissue, indicated that they were well tolerated by the tissues, therefore, MTA / CHG seemed to be biocompatible.
Control group of sterile water showed no inhibition and control groups of Chlorhexidine always were inhibitory. It showed a good response by inhibiting the growth. From these findings it can be concluded that the Chlorhexidine in the MTA mixture was producing the enhanced antimicrobial action.
It is imperative for the bactericidal material to be harmful to the bacterial cells or otherwise since targeted antibacterial/cytotoxic effect is still an illusive phenomenon. The antibacterial substances do have their cytotoxic effects which are beyond the purview of the organization of the present study.
Thus, to conclude in this in vitro study substitution of 0.12% Chlorhexidine gluconate for sterile water in ProRoot Mineral Trioxide Aggregate increased the antimicrobial effect.
This present study analyzed only the antimicrobial efficacy of Mineral Trioxide Aggregate/Chlorhexidine in Ex-vivo environment, further studies are of essential paramount before one could recommend the combination of Mineral Trioxide Aggregate/Chlorhexidine for clinical use.
Conclusion
The following conclusion is arrived from the present ex-vivo study:
Mineral Trioxide Aggregate with Chlorhexidine exhibited greater antimicrobial efficacy than Mineral Trioxide Aggregate with water against all the microorganisms used in this study.
The ex-vivo settings in the present study preclude the many variables found in the actual clinical situation because of the differences in the physical state (e.g. setting and working times). Considering the results of the present investigation, Chlorhexidine has several properties that suggest it can be a suitable alternative to water to be mixed with Mineral Trioxide Aggregate, but further research is required to assess various other aspects.
References
1. Jose F. Siqueira, Milton de Uzeda: Evaluation of the antibacterial effects of Chlorhexidine, Metronidazole, and Calcium hydroxide associated with three vehicles.J.O.E. 1997; 23(3): 167-169.
2. Goran Sundqvist, Umea Sweden:Taxonomy, Ecology and Pathogenicity of the Root Canal Flora. Oral Surg Oral Med Oral Pathol 1994; 78: 522-530.
3. Goran Sundqvist, David Figdor, Sten Persson, Ulf Sjogren:Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol 1998,85, No.1 Jan. 86-93.
4. Mahmoud Torabinejad, Noah Chivian: Clinical applications of Mineral Trioxide Aggregate. J.O.E. 1999; 25(3): 197-205.
5. Delany G.M., Patterson S.S., Miller C.H., Newton C.W.:The effect of Chlorhexidine gluconate irrigation on the root canal flora of freshly extracted necrotic teeth. Oral Surg Oral Med Oral Pathol 1982; 53: 518-523.
6. M. R. Leonardo, M. Tanomaru Filho, L.A.B. Silva, P.Nelson Filho, K.C. Bonifacio, I.Y.Ito:Antimicrobial Activity of 2% Chlorhexidine used as a root canal irrigating solution. J.O.E. 1999; 25(3): 167-171.
7. M. Torabinejad, C.U. Hong, T.R. Pitt Ford, J.D. Kettering:Antibacterial Effects of some Root End Filling Materials.J.O.E. 1995; 21(8): 403-406.
8. Mahmoud Torabinejad, C.U. Hong, F. McDonald, T.R. Pitt Ford: Physical and chemical properties of a new root end filling material. J.O.E. 1995; 21: 349-353.
9. Marcela Fridland, Rafael Rosado: Mineral Trioxide Aggregate solubility and porosity with different water to powder ratios. J.O.E.; 29(12): 814-817.
10. Khalid Al-Hezaimi, Khalid AL-Hamdan, Jafar Naghshbandi, Samuel Oglesby, James H.S. Simon, Ilan Rotstein.: Effect of white colored MTA in different concentrations on Candida albicans in vitro.J.O.E. 2005; 31(9): 684-686.
11. Ayce Unverdi Eldeniz, Hasan Huseyin Hadimli, Hanife Ataoglu, Dag Orstavik: Antibacterial Effect of selected root-end filling materials. J.O.E. 2006; 32(4): 345-349.
12. Khalid Al-Hezaimi, Thakib A. Al-Shalan, Jafar Naghshbandi, Samuel Oglesby, James H.S. Simon, Ilan Rotstein:Antibacterial Effect of Two Mineral Trioxide Aggregate (MTA) Preparations against Enterococcus faecalis and Streptococcus sanguis. J.O.E. 2006; 32(11): 1053-1056.
13. Dystein Fardal, Robert S. Turnbull:A review of the literature on use of Chlorhexidine in dentistry. JADA 1986; 112 (6): 863-869.
14. V. Peciuliene, A.H. Reynaud, I. Balciuniene, M. Haapasalo:Isolation of yeasts and enteric bacteria in root filled teeth with chronic apical periodontitis. International Endodontic Journal 2001; 34: 429–434.
15. J.W. Ferguson: Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J.O.E. 2002; 28(2): 68-71.
16. Khalid Al-Hezaimi, Jafar Naghshbandi, Samuel Oglesby, James H.S. Simon, Ilan Rotstein: Comparison of Antifungal Activity of White-Colored and Gray-Colored Mineral Trioxide Aggregate (MTA) at Similar Concentrations against Candida albicans.J.O.E. 2006; 32(4): 365-367.
17. Mahmut Sumer, Mehtap Muglali, Emre Bodrumlu, Tolga Guvenc:Reactions of connective tissue to Amalgam Intermediate restorative material, MTA and MTA mixed with Chlorhexidine. J.O.E. 2006; 32(11): 1094-1096.
|