INTRODUCTION
Diphenylhydantoin (DPH) was introduced into clinical practice in 1938 and; since then, has been the preferred drug in the treatment of seizure – disorders. As patients frequently have to take this drug for many years, it is not surprising that
several side effects have been reported. Gingival – enlargement is one of the first side effects of DPH reported by Kimball (1939)19 and occurs in some patients. Studies on both human and animals have shown that, although plaque accumulation
and the associated inflammation may be necessary for the initiation of gingival – hyperplasia, 21, 23, 26, 30 their presence does not mean lesion development is inevitable (Ishikawa et al 1961)15. There is general agreement that the gingival hyperplasia seen in phenytoin patients is the result of an increase in amount of connective tissue rather than a marked epithelial hyper plasia 7, 13, 17, 24. Though reports in literature suggest that the major change in phenytoin gingival hyperplasia may be due to an increase in the amount of collagen, it is un-clear whether this increase is associated with an increase in the number of fibroblast 3, 13, 17, 27 or with elevated cellular activity 4, 16, 36 or with both. Hall and Squier12 demonstrated increase in ground substance and suggested that the relative increase in ground substance may be due to decrease breakdown of extra cellular material within fibroblasts, while synthetic activity is maintained at a constant level. However, there is agreement from such studies that connective tissue degradation is decreased in the presence of phenytoin11, 32. Goultschir & Shoshan (1980)11 have observed that phenytoin inhibits collagen degradation in the gingival tissue and fibroblast populations differ in production of active and total collagenase enzyme (Hurum et al 1982)14. Substantial evidence suggests that one factor participating in collagen destruction is collagenase, the enzyme needed for specific degradation of collagen in vivo (Robertson et al 1976) 31. Eisen and coworkers (1970)9, (1971)10, identified alpha-2 macro globulin and alpha-1antitrypsin, the two main collagenase inhibitors in serum. The interaction of gingival collagenase
and serum alpha-2 macro globulin was further studied by Abe and Nagai (1972)1, and Birkedal – Hansen et al (1975)5 who demonstrated the formation of a tight enzyme – inhibitor complex, which has retained no collagenolytic activity.
Therefore, the present study is proposed to compare the serum and salivary concentrations of alph a-2 macroglobulin and alpha-1 antitrypsin between non-phenytoin patients with normal gingival, and phenytoin patients associated with varying degrees of gingival – enlargement, andto study the possible relationships of these inhibitors to varying degrees of gingival
enlargement in order to elaborate our present knowledge on the biochemical aspect of tissue changes in phenytoin gingival enlargement.
REVIEW OF LITERATURE
The purpose of this review is to summarise the current state of knowledge concerning the pathogenesis of phenytoin induced gingival hyperplasia.
(a) Factors affecting phenytoin induced gingival hyperplasia :
(i) Role of Plaque :
King (1954)20, Panuska et al (1961)29 and Kerr (1952)18 have made an association between poor oral hygiene and most advanced stage of phenytoin hyperplasia. Steinberg and Steinberg (1982)35 studied the distribution, severity and control of phenytoin induced gingival over growth (PIGO) in 19 severely retarded children, from 4 to 15 years of age, 16 of the patients were mouth breathers. They observed some degree of PIGO, 11 % had grade II and 37% had a grade- I. They concluded that in severely retarded individuals where only limited oral care can be performed, use of topical agents such as SnF2 and use of an electric tooth brush may aid in plaque control and therefore help to control PIGO.
(ii) Role of inflammation :
Nuki and Cooper 28 were able to induce gingival overgrowth in 11 of 12 PHT – treated cats in those areas of gingival where localirritation created inflammation; conversely the non irritated, non inflamed gingival in the same animals showed no over growth. Church and Dolby (1978) 6 studied the effect of Dilantin on the cellular response to dento gingival plaque extract. They suggested that Dilentin may reduce the components of immune system like cytotoxic factor produced by lymphocytes which is damaging for fibroblast, or reduced the production and secretion of collagenase and other hydrolyzing enzymes produced by
macrophages capable of breaking connective tissue and so contribute towards a hyperplastic inflammatory response of the gingival. Thus inflammation seems to be an important co-factor in PHT – induced gingival over growth in both animals and men.
(iii) Alteration in composition of hyperplastic gingiva :
Dahllof et al (1986)8 studied proteoglycans and glyclosaminoglycans composition in normal gingiva and phenytoin induced
gingival over growth. They noted in normal gingiva, 0.8% uronic acid per dry tissue weight, while in phenytoin induced gingival
over growth, there was markedly increased amount of uronic acid, 2.1% compared to normal gingival. The collagen content was
relatively decreased in the phenytoin lesion. Hall and Squier (1982) 12 studied the effect of phenytoin (PHT) on inflamed as well as non inflamed gingiva in order to study the role of inflammation and drug in the development of PHT-induced gingival over growth in the ferrets. They concluded that the relative increase in ground substance may reflect decreased breakdown of extracellular material within fibroblasts, while synthetic activity was maintained at a constant level.
(iv) Change in collagen metabolism:
Schneir et al (1978) 34 compared the phenotype and degree of hydroxylation of collagen chains solubilised from over grown tissue to chains isolated from inflamed gingival. The tissues were obtained from the DPH- treated patients in the ages of 17, 27, 31, 35 and 42, whereas the mean age of patients receiving routine therapy was 46 ears. Their results showed that the amount of hydroxyproline in gingiva obtained from DPH – treated patients was less than that in the gingiva from patients with inflamed gingiva. Narayan et al (1988) 25 studied regulation of collagen production by fibroblasts cultured
from normal and phenytoin induced hyperplastic gingiva. They obtained fibroblasts from clinically healthy human gingival tissues and from phenytoin induced hyperplastic gingiva. They concluded that collagen production in gingival fibroblasts was
primarily regulated by the mRNA levels and that over production of collagens by cells from phenytoin induced hyperplastic gingiva was resulted from an increased steady state level of collagen mRNA and not decreased collagen degradation.
(b) Collagenase inhibitors:
Abe and Nagai (1972) 1 studied interaction between tadpole collagenase and human alpha-2 macroglobulin by employing
immunoadsorbent chromatography and gel filteration. Collagenase activity was assayed by measuring the release of 14 C- labeled peptides from a reaction mixture containing enzyme and native reconstituted guinea pig skin collagen fibrils. It was found that native alpha-2 macroglobulin irreversibly binds tad pole collagenase at neutral ph; in activating the enzyme, and the complex did not dissociate until the globulin was denatured. Schenkein and Genco (1977) 33 evaluated the alpha-1 antitrypsin and alpha-2 macroglobulin in serum and gingival crevicular fluid by single radial – immunodiffusion method. The gingival fluid and serum samples were collected from 18 subjects; all showed chronically inflamed gingiva. The gingival fluid to serum ratio of alpha-1 antitryptsin and alpha-2 macroglobulin was found to be 71.4% and 65.7% respectively, which appeared to represent a minute dilution of serum and, that sever periodontal damage may produce a more diluted gingival fluid. There is general agreement that the gingival hyperplasia seen in phenytoin patients is the result of an increase in amount of gingival connective tissue rather than a marked epithelial hyperplasia. A survey of literature presents sufficient evidence to show that protease inhibitors reduces the collagenase enzyme activity and in turn reducing the total degradation of collagen and collagen substance, while the rate of collagen and ground substance synthesis may remain unchanged by phenytoin.
MATERIAL AND METHODS
Four groups of 50 patients were included in this study in the age group of 11 – 45 years. Group-I (Control) was comprised of 10
individuals (5 male, 5 female, mean age 23) with no history of epileptic seizures and phenytoin ingestion. Group-II consisted of 10 epileptic patients (5 male, 5 female, mean age 28) who had been receiving phenytoin for a minimum of 1 year and without gingival enlargement. Group-III included 16 epileptic patients (6 male, 10 female, mean age 23) with grade-1 phenytoin associated gingival enlargement, and Group-IV consisted of 14 epileptic patients (8 male, 6 female, mean age 21) with grade-II phenytoin associated gingival enlargement. The degree of gingival enlargement was assessed by established clinical criteria on 0- 3 scale (Angelopoulos and Goaz 1972) 2.
Epileptic patients were selected from out patient Department of Neurology and Psychiatry, Kasturba Medical College, Manipal. The control group was selected from the out patient Department of College of Dental Surgery, Manipal, on the basis of negative history of epilepsy or phenytoin medication.
Clinical Assessment :
Gingival inflammation was assessed by Gingival Index of Loe and Silness (1963) 22 as follows :-
SCORE CRITERIA
0 Normal gingiva.
1 Mild inflammation, slight change in colour, slight oedema, no bleeding on palpation.
2. Moderate inflammation, redness, oedema, glazing, bleeding on palpation.
3. Severe inflammation, marked redness, oedema, ulceration, tendency to spontaneous bleeding.
The Gingival hyperplasia was graded by using Angelopoulos and Goaz (1972) 2 criteria;
0 No hyperplasia, normal gingiva, crest of marginal gingiva at CEJ.
1 Hyperplastic gingiva, extending the cervical third or less of the anatomic crown.
2. Hyperplastic gingiva extending more than cervical third but less than middle third of anatomic crown.
3. Hyperplastic gingiva extending more than two-third of the anatomic crown.
On the basis of gingival enlargement index phenytoin treated patients were divided into three groups by using following score :-
Parameters Gingival enlarge-ment score
(i) Group of 10 non-phenytoin patients — with normal gingiva was considered as the control group ( Group-I)
(ii) Group of 10 phenytoin patients with 0.0 to 0.2normal gingiva (Group-II)
(iii) Group of 16 phenytoin patients with 0.3 to 1.1 mild gingival enlargement (Group-III)
(iv) Group of 14 phenytoin patients with 1.2 to 2.0 moderately severe gingival enlargement (Group-IV)
Collection of samples :
5 ml. of unstimulated whole saliva was collected in a sterile test tube following a brief rinsing of mouth with water. 6 ml. of blood was collected from the median cubital vein of each patient by using a sterile glass syringe and a disposable needle (22 gauze) into a sterile test tube. The samples were taken immediately to the clinical Biochemistry Laboratory, KMC, Manipal for an estimation of alpha-1 antitrypsin and alpha- 2 macroglobulin in both saliva and blood by spectrophotometric method.
Results :
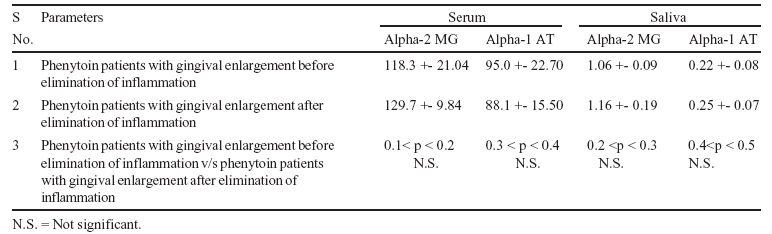
It was observed that alpha-2 macroglobulin concentration in serum and saliva of nonphenytoin patients with normal gingiva was within normal limits and concentration in serum and saliva of phenytoin patients with gingival enlargement was found to be increased after the elimination of inflammation (Table-3). It was observed that alpha-1 antitrypsin concentration in saliva of non-phenytoin patients with normal gingiva was within normal limits. The concentration decreased in phenytoin patients with
normal gingiva but increased again in phenytoin patients with mild and moderately severe gingival enlargement. It was also observed that alpha-1 antitrypsin concentration in the serum of phenytoin patients with gingival enlargement was found to be
decreased after the elimination of inflammation, while in saliva of same patients, the concentration of alpha-1 antitrypsin was found to be increased after the elimination of inflammation (Table-3).
SUMMARY AND CONCLUSION
The present study was attempted to compare the serum and salivary concentration of alpha-2 macroglobulin and alpha-1 antitrypsin between non-phenytoin patients with normal gingiva and phenytoin patients with varying grades of gingival
enlargement; and to co-relate serum and salivary concentration of alpha-2 macrogloblin and alpha- 1 antitrypsin among phenytoin patients with varying grades of gingival enlargement. Samples and blood and saliva were collected
from 50 patients belonging to 4 groups. Group-I consisted 10 non-phenytoin patients with normal gingiva ( control group ), Group-II consisted 10 phenytoin patients with normal gingiva, Group- III consisted 16 phenytoin patients with mild gingival enlargement and Group-IV consisted 14 phenytoin patients with moderately severe gingival enlargement. Samples of both blood and saliva were subjected for quantitative estimation of alpha-2 macroglobulin and alpha-1 antitrypsin by
spectrophotometer. Results obtained have been presented in Table-1, Table-2 and Table-3 above. From the analysis of the results, the following conclusions were drawn :-
(i) The mean serum and salivary concentrations of both Alpha-2 macroglobulin and Alpha-1 antitrysin were found to be significantly decreased in phenytoin patients with varying grades of gingival enlargement compared to non phenytoin patients with normal gingiva.
(ii) When mean concentration of Alpha-2 macroglobulin and Alpha-1 antitrypsin in serum and saliva were compared among
phenytoin patients with varying grades of gingival enlargement, no significant differences were found.
(iii) No statistical significant co-relation was found between mean gingival enlargement score and mean concentration of Alpha-2 macroglobulin and Alpha-1 antitrypsin in serum and saliva of phenytoin patients with varying grades of gingival enlargement.
(iv) The mean serum and salivary concentrations of Alpha-2 macroglobulin in both serum and saliva of phenytoin patients with gingival enlargement was found to be increased after the elimination of inflammation, while mean serum concentration of Alpha-1 antitrypsin was found to be decreased after the elimination of inflammation, but the differences were not statistically significant.
(v) The possible reasons for decreased Alpha-2 macroglubulin and Alpha-1 antitrypsin concentration in both serum and saliva of
phenytoin patients with varying grades of gingival enlargement are discussed.
TABLE - 1: Mean concentrations of Alpha-2 Macroglobulin and Alpha-1 Antitrypsin in serum and saliva of non-phenytoin patients
with normal gingiva and phenytoin patients with normal gingiva and varying grades of gingival enlargement.
TABLE-2: Statistical significance between mean concentrations of Alpha-2 Macroglobulin and Alpha-1 antitrypsin in serum and
salyba of non-phenytoin patients with normal gingiva and phenytoin patients with varying grades of gingival enlargement.
TABLE- 3: Mean concentrations and statistical significance of Alpha-2 Macroglobulin and Alpha-1 antitrypsin in serum and saliva of phenytoin patients with gingival enlargement (Grade-I & Grade-II ) before and after elimination of inflammation.
 |
 |
REFERENCES
1. Abe, S. & Nagai, Y. (1972) : Interaction between tadpole collagenase and human Alpha-2 macroglobulin. Biochem,
Biophys. Acta. 278 : 125 - 132.
2. Angelopoulos, A. P & Goaz, P. W. (1972) : Incidence of diphenylhydantoin gingival hyperplasia. Oral Surg. 34 : 898
– 906.
3. Angelopoulos, A.P (1975) : Diphenylhydantoin gingival hyperplasia : a clinicopathologic review. J.Can. Dent. Assoc.
41 : 103 – 106.
4. Bienkowski, R.S. (1983) : Intracellular degradation of newly synthesized secretory protein. Biochem. J.83 : 214 : 1.
5. Birkedal, H.H., Cobb, C.M., Taylor, R.E., and Fullmer, H.M. (1975) : Serum inhibition of gingival collagenase. J. Oral
Path. 3 : 284 – 290.
6. Church, H.A., and Dolby, A.E. (1978) : The effect of Dilantin on the cellular immune response to Dento – gingival plaque
extract. J.Periodontol. 49 : 373 – 377.
7. Ciancio, S.G., Yaffe, S.J. and Catz, C.C. (1972) : Gingival hyperplasia and Diphenyl hydantoin. J. Periodontol. 43 : 411
– 414.
8. Dahllof, G., Modeer, T., Reinholt, F.P., Wikstrom, B., and Hjerpe, A. (1986) : Protioglycans and Glycoseaminoglycans
in phenytoin – induced gingival over growth. J.Periodont. Res. 21 : 13 – 21.
9. Eisen, A.Z., Bloch, K.J. and Sakai, T. (1970) : Inhibition of human skin Collagenase by human serum. J.Lab. Clin. Med.
75 : 258 – 263.
10. 10. Eisen, A.Z., Baner, E.A. and Jeffrey, J.J. (1971) : Human skin Collagenase. The role serum alpha-globulins in the control of activity in vivo and in vitro. Pros. Nat. Acad. Sci. 68 : 248 – 251. Cited by Birkedal, H.H., Cobb, C.M., Taylor, R.E.,
and Fullmer, H.M. (1975) : Serum inhibition of gingival collagenase. J.Oral Path. 3 : 284 – 290.
11. Goultschin, J.and Shoshan, S. (1980) : Inhibition of collagenase breakdown by Diphenylhydantoin. Biochem.
Biophys. Acta. 631 : 188 – 191.
12. Hall, B.K., and Squier, C.A. (1982) : Ultrastructural quantitation of connective Tissue changes in phenytoin –
induced gingival over growth in the ferret. J.Dent. Res. 61 (7) : 942 – 952.
13. Hassell, T.M. and others (1976) : Diphenylhydantoin (Dilantin) gingival Hyperplasia : drug induced abnormality of connective
tissue. Proc. Nat. Acad. Sci. USA 73 : 2909 – cited by Keith, D.A., (1968) : Side effects of Diphenylhydantoin : A review.
J. Oral Surg. 36 : 206 – 209.
14. Hurum, S., Sodek, J., Abubin, J.E. (1982) : Synthesis of collagen, collagenase and Collagenase inhibitors by cloned
human gingival fibroblasts and the effect of Concanavalin A. Biochem. Biophys. Res. Commun. 107 : 357. Cited by Narayanan, A.S. Meyers, D.F., Page, R.C. (1988) : Regulation of collagen production in fibroblasts cultured from normal and phenytoin induced hyperplastic human gingiva. J. Periodont. Res. 23 : 118 – 121.
15. Ishikawa, H and Glickman, I. (1961) : Gingival response to the systemic Administration of sodium diphenylhydantoin (Dilantin ) in cats. J. Periodontol. 32 : 149 – 158. Cited by Hall, B.K. and Squier, C.A. (1982) : Ultrastructural quantitation of
connective tissue changes in phenytoin induced gingival over growth in the ferret. J. Dent. Res. 61 (7) : 942 – 952.
16. Kantor, M.L., and Hassel, T.M. 1983 : Increased accumulation of sulfated Glycoseaminoglycans in cultures of human
fibroblasts from phenytine induced gingival over growth. J. Dent. Res. 62 (3) : 383 – 387.
17. Keith, D.A., Paz, M.A. and Gallop, P.M. (1977) : The effect of diphenylhydantoin on fibroblasts in vitro. J.Dent. Res. 56
(10) : 1279 – 1283.
18. Kerr, D.A. (1952) : Stomatitis and gingivitis in the adolescent and the pre-adolescent. J.Am. Dent. Assoc. 44 : 27 – 34.
19. Kimball, O.P. (1939) : Treatment of epilepsy with sodium diphenylhydantoin. JAMA 112 : 1244 – 1245. Cited by Sklams, S., Taylor, R.G. and Shkar, G. (1967) : Effect of diphenylhydantoin sodium on healing of experimentally produced fractures in rabbit mandibles. J. Oral Surg. 25 : 311– 319.
20 King, J.D. (1954) : Experimental and clinical observations on gingival hyperplasia due to diphenylhydantoin. Brit. Dent. J.
96 : 237 – 248.
21. King, D.A., Hawes, R.R. and Bibby, B.G. (1976) : The effect of oral physiothraphy on Dilantin gingival hyperplasia. J. Oral
Path. 5 : 1 – 7.
22. Loe, H., Silness, J. (1963) : Periodontal disease in pregnancy : I. Prevalence and severity. Acta. Odon. Scand. 21 : 533 –
551. Cited by O’Neil, T.C.A. and Figures. K.H. (1982). The effects of chlorhexidine and mechanical methods of plaque
control on the recurrence of gingival hyperplasia in young patients taking phenytoin. Br. Dent. J. 152 : 130 – 133.
23. Modeer, T., and Dahllof, G. (1987) : Development of phenytoin induced gingival over growth in non-institutionalised
epileptic children subject to different plaque control programs. Acta. Odont. Scand. 45 : 81 – 85.
24. Narayanan, A.S. and others (1983) : Biosynthesis and regulation of type-IV collagens in Diploid human fibroblasts.
J. Biol. Chem. 258 : 11694.
25. Narayanan, A.S., Meyers, D.F., Page, R.C. (1988) : Regulation of collagens production in fibroblasts cultured from normal
and phenytoin induced hyperplastic human gingiva.J.Periodont. Res. 23 : 118 – 121.
26. Nascimento, A.D., Barreto, R.D.C. , Bozzo, L. and Almeida, O.P. (1985) : Interaction of phenytoin and inflammation induces gingival over growth in rats. J.Periodont. Res. 20 : 386 – 391.
27. Noess, T. (1969) : The effect of 5, 5 – Diphenylhydantoin (Dilantin) on fibroblast – Like cells in culture. J.Periodont.
Res. 4 (2) : 163 – 164.
28. Nuki, K. and Cooper, S.H. (1972) : The role of inflammation in the pathogenesis of gingival enlargement during the
administration of diphenyhydantoin sodium in cats. J. Periodont. Res. 7 : 102. |