Introduction
Carcinoma of the oral cavity is one of the commonest malignant neoplasms seen in North India. Ironically it is preventable to a large extent by cessation of betel nut – tobacco chewing and smoking. Oral cancer is often preceded by premalignant lesions. There are well-recognized premalignant lesions especially leukoplakia and erythroplakia which have a 5-10% malignant transformation rate[1]. Leukoplakia may range microscopically from benign hyperkeratosis to invasive squamous cell carcinoma. Mutations of the tumor suppressor gene p53 seem to be largely associated with many oral cancer cases. Its expression in premalignant lesions like oral epithelial dysplastic conditions has not received equivalent attention till now. Immunohistochemical analysis of biopsy tissue (alteration in tumor suppressor gene p53 and its expression) may prove to be useful prognostic biomarker and may also aid in diagnosing recurrence at early stage when in comparison with traditional method. This is important because detection of p53 mutation could predict malignant transformation in dysplastic lesions and thus may prove as a guide to early prophylactic treatment. So one of the approaches to control oral cancer is to detect oral precancerous lesions early in development and prevent its malignant transformation and if recurrence occurs that should also be detected at the earliest to avoid dire consequences. Although in recent years, ample of studies have been available discussing the role of p53 gene as a diagnostic measure in cancer lesions but very few have tried to focus on premalignant lesions like leukoplakia and the worst part comes when the recurrence takes place after treatment. So we decided to conduct a trial analysing the role of p53 immuno-histochemical analysis in diagnosis and early detection of recurrences in 30 oral leukoplakic patients.
Materials and Methods
We studied 30 clinically proven leukoplakic patients who presented to the Department of Oral & Maxillofacial Surgery, Sudha Rustagi Dental College, Faridabad, Haryana for their management between August 2013 to November 2013 and were followed over a period of 1 year to detect the recurrences. All patients provided written informed consent before enrolling. The study was conducted in accordance with the Declaration of Helsinki, as updated, and was approved by the local ethics committee. Demographic details were recorded for all the patients in which both the genders were included and the age group ranged from 30-60 years of age. All the patients suffering from lichen planus, oral submucous fibrosis, or any other white lesion were excluded. Surgical excision of the lesion was performed as a treatment modality and the biopsy specimen was divided into two equal halves for diagnosis using p53 immunohistochemical analysis and histopathological examination. Postoperatively punch biopsy specimens were obtained from the same site of operation at 2 intervals- 6 month and 1 year to detect the recurrences using the same diagnostic measures.
 | Table – 1: Diagnosis Of Leukoplakia By Histopathology And P 53 Level
 |
 | Table – 2 Post-operative (6 Months) Examination By Histopathology And P 53 Level For Recurrence
 |
Immunohistochemistry
For detection of p53 protein, APES technique was used. Formalin fixed, paraffin embedded biopsy tissue specimens were cut into 5 µm sections and fixed on slides coated with poly – L – lysine. The subsequent steps of the immunohistochemistry procedure were as follows: Tissue sections were de-paraffinized in xylene and rehydrated by passing through graded alcohol. The sections were then placed in 100 ml citrate buffer, pH 6.0 and were then micro waved at 750 W for 15 minutes. After washing with water, the sections were incubated in 3% hydrogen peroxide for 30 minutes to inactivate endogenous peroxidase activity. After rinsing with Tris – buffered saline (TBS), the sections were incubated in the primary antibody (anti p53 antibody) for 1 hour at room temperature. The primary antibody was used in a dilution of 1:50. After washing in TBS, sections were incubated with biotinylated secondary antibody for 30 minutes and then after washing; sections were incubated in diaminobenzidine solution for 10 minutes. The sections were washed in water, counterstained with hematoxylin and mounted.
Immunoscoring for p53
Clear brown nuclear staining evidenced positive staining. Cytoplasmic staining was not considered. Only nuclear stain was considered positive. The intensity was graded as: grade 0: no labelling with regard to dysplastic changes; grade I: weak or equivocal staining, grade 2: unequivocal positive labeling. The p53 count index was expressed as number of positive cells per 1000 dysplastic cells. When more than 10% nuclei showed staining it was taken as positive for p53 protein. If less than 10% nuclei took up stain the result was considered as equivocal [2].
Results
Of 30 leukoplakic patients, mean age group of the patients included were 30-60 years. All the patients had a history of smoking and/or tobacco chewing. 25 were males and 5 were females. Data was stored in a Microsoft excel database and analyzed with SPSS version-11 statistical package with Continuous variables given as mean (SD), median and range. Chi- square test with 95% CI and p<0.05 was considered significant.
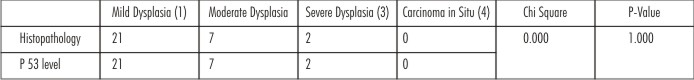
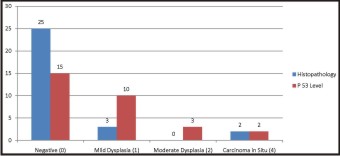
Table 1 & Graph 1 reflect the histopatho logical and immunohistochemical analysis of 30 tissue specimens at diagnostic level after the surgical excision of diseased part. No statistically significant difference was observed in both the analysis (p> 0.05) and a strong correlation could be observed between them. 70 % of the patients presented with mild dysplasia and grade 1 p 53 scoring. 7 (23%) patients presented with moderate dysplasia and grade 2 p53 scoring and only 2 (6.67%) had severe dysplastic changes & grade 3 p53 scoring. No patients with carcinoma in situ were recorded at the diagnostic level.
 | Table – 3 Post-operative (1 Year) Examination By Histopathology And P 53 Level For Recurrence
 |
 | Graph 1 - Diagnosis Of Leukoplakia By Histopathology And P 53 Level
 |
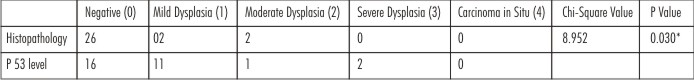
Table 2 & Graph 2 present the histopatho logical and immuno histochemical analysis of the biopsy specimen from same operational site at the time interval of 6 months postoperatively. 26 (86.7%) patients presented with negative results on histopathological examination whereas only 16 (53.3%) patients showed negative results with immunohistochemical analysis. Only 2 (6.67%) patients had mild dysplasia whereas 11 (36.7%) patients had grade 1 scoring on immunohistochemical analysis. Moderate dysplasia was seen in 2 patients only and 1 patient was observed with grade 2 scoring. Although none of the cases were reported with severe dysplasia or carcinoma in situ on histopathological analysis, 2 (6.7%) patients presented with grade 3 scoring on immunohistochemical analysis. Chi square analysis shows significant difference presenting the earlier detection of dysplastic changes with p53 immunohistochemical analysis. (p<0.05)
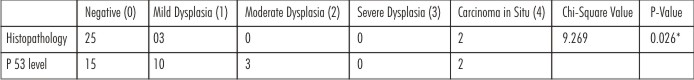 | Graph 2 - Post-operative (6 Months) Examination For Recurrence Of Leukoplakia By Histopathology And P 53 Level
 |
 | Graph 3 - Post-operative (1 Year) Examination For Recurrence Of Leukoplakia By Histopathology And P 53 Level
 |
Table 3 & Graph 3 represent the histopatho logical & immuno histochemical analysis at the time interval of 1 year postoperatively to detect the recurrence.25 (83.3%) patients had negative report on histological evaluation whereas only 15 patients(50%) had negative report on immunohistochemical evaluation. 3 (10%) patients showed mild dysplastic changes whereas 10(33.3%) patients had grade 1 scoring for p53 levels. None of the patients had moderate dysplastic changes on histological analysis whereas 3 (1%) patients had grade 2 scoring on p53 analysis. 2 (6.67%) patients each presented carcinoma in situ changes and grade 4 scoring for p53. So the statistically significant difference was present on comparison of histopatho logical and immuno histochemical analysis at the time interval of 1 year. (p<0.05)
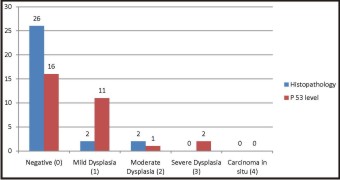 |
 |
 |
 |
Discussion
The p53 gene consists of 16 to 20 kilobases of DNA on the short arm chromosome 17 at position of 17p 13.1 [3]. It was earlier considered to be a dominant oncogene as its over-expression led to immortalization of rodent cells. Now the p53 is considered to be a tumor suppressor gene.
The protein product of p53 gene is a 393-aminoacid nuclear phosphoprotein with a molecular weight of 53 KD. The normal or wild type of p53 protein has a short half-life and is therefore present in very minute quantities making it undetectable by conventional immunohistochemical staining. Mutation of p53 gene results in abolition of its tumor suppressor activity. The protein product of the mutated gene exists in a stable form and can accumulate in tissues making it recognizable by immunohistochemical techniques [4].
Mutation of p53 gene is one of the most common events in carcinogensis including squamous cell cancers of the head and neck[5]. Accumulation of p53 protein has been detected in premalignant lesions of esophagus, bronchus and also oral leukoplakia[6] and thus the purpose of this study was to assess the efficacy of p53 immunohistochemical analysis in diagnosing and detecting recurrences in leukoplakic patients at short time interval.
Cancer of the oral cavity is the best possible model for studying the oncogenic potential of p53 gene mutation but simultaneously the study of oncogenic potential in premalignant conditions may aid in reducing its malignant transformation. One of the well-known premalignant lesions, which is most usually observed, is leukoplakia with dysplastic changes. The oral cavity can be visually inspected and suspicious lesions directly palpated and biopsied. The patient can also be easily followed up and recurrent diseases can be clinically diagnosed without resorting to expensive imaging techniques. All these factors make oral leukoplakic lesions the ideal model for the study of p53 gene mutation in oncogenetic changes.
We found all the 30 oral leukoplakic specimens took p53 protein staining on immunohistochemistry. Of these, 21 patients had grade 1 or equivocal or weak stain & 7 had grade 2 or unequivocal positive stain while only 2 had grade 3 score. On histopathological examination, all the 30 patients had evidence of dysplasia. This could be well correlated as grade 1 score with mild dysplastic changes and further. Finding of p53 protein product in dysplastic leukoplakia specimens suggests that p53 gene mutation may be a crucial event in malignant transformation of oral dysplastic leukoplakia lesions and thus can serve as a diagnostic biomarker.
Discrepancies were experiential between p53 protein detection by IHC and p53 gene mutation detection on sequencing. Both may not be co-existent in the same patient and p53 protein positive immunostaining may occur without p53 gene mutation[7]. The reverse situation has also been described by p53 gene mutation occurring without p53 protein accumulation. The reason attributed to this phenomenon is that p53 gene mutation may be a gross deletion, which abolishes p53 protein production. Conversely p53 protein identification without gene mutation is ascribed to the fact that normal p53 protein may be present in large quantities or become stabilized and therefore detectable on IHC. Human papilloma virus type 16 & 18 are known to stabilize p53 protein [8]. Thus p53 protein detection does not necessarily signify p53 gene mutation and p53 gene mutation may sometimes not translate into p53 protein identification. Even in our study, after the surgical excision of the lesion has been performed, at the interval of 6 months, 26 patients did not show any dysplastic changes whereas amongst these only 16 patients has presented with nil p53 expression.
Earlier p53 protein detection was associated with invasive tumors. Recent studies have started documenting p53 protein in preinvasive lesions of esophagus, bronchus and oral cavity[6]. We also found p53 protein expression in all of the 30 leukoplakia specimens but may present with false positive results. Other studies have reported more than 75% dysplasias showing over expression of p53 protein[4]. The results between different studies may vary due to different techniques and antibodies or different tissue processing techniques. The presence of p53 over expression in oral dysplastic lesions suggests that p53 gene mutation is an important and early genetic event in oral carcinogenesis and thus can prove to be a biomarker in diagnosis
It has been emphasize that immuno histochemical identification of p53 almost certainly implies a protein with a defective function because of either mutation or inactivation [9]. Kumar et al., found that expression of p53 and Ki-67 increased with increasing grades of dysplasia in leukoplakia specimens indicating it to use as predicative markers in oral cancer development [10]. Even the expression of p53 when correlated significantly with Ki-67 is suggestive of altered p53 gene product promoted cellular proliferation. In another study on oral premalignant lesions the labeling index for p53 ranged from 7.9 to 71.9 in oral submucous fibrosis and 65.2 to 85.9 in oral squamous cell carcinoma[11]. It was suggested that p53 expression could be used as a surrogate marker of malignant transformation in oral submucous fibrosis but until now, none of the studies has focussed on early detection of recurrences in leukoplakic lesions and thus stopping the malignant transformation. In our study, two patients that did not present severe dysplastic changes showed greater p53 scoring at 6 months interval developed carcinoma in situ at 1-year interval.
There is the clinical possibility of using p53 protein detection by IHC in oral dysplastic lesion biopsies for identifying premalignant lesions at high risk for malignant conversion preoperatively as well to check the recurrences postoperatively. Currently there is no reliable method of identifying this sub-group at high risk. Patients of dysplasia showing p53 protein expression in their biopsies could be put on a rigorous follow-up program after wide local excision of their lesions.
Conclusion
On immunohistochemistical analysis of p53 protein in all the leukoplakic lesions, it proved to be a biomarker in early diagnosis for further dysplastic changes. On postoperative evaluation after the surgical excision of lesion in all the 30 cases, in 2 cases with p53 staining could present the malignant transformation 6 months earlier than dysplastic changes visible on histopathological examination. These findings suggest that p53 gene mutation could be an important event in oral carcinogenesis and in early detection of recurrence. Further, in oral dysplastic lesions, expression of p53 protein could indicate high risk for malignant conversion of the lesion but to avoid bias by false positive results we need more number of randomized controlled trials with larger sample size to achieve definite outcomes.
References
1. Daftary DK, Murti PR, Bhonsle RR, Gupta PC, Mehta FS, Pindborg JJ. Risk factors and risk areas of the world. In Johnson NW, editor. Oral Cancer: the detection of patients and lesions at risk. Cambridge UK: Cambridge University Press. 1991:29 -63.
2. Kamel D, Pääkkö P, Nuorva K, Vähäkangas K, Soini Y. p53 and c-erbB-2 protein expression in adenocarcinomas and epithelial dysplasias of the gall bladder. J Pathol. 1993; 170(1):67-72. [Pubmed].
3. McBride OW, Merry D, Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Pro NatlAcadSci USA 1986; 83: 130 – 4. [Pubmed] [Full text]
4. Rowley H, Sherrington P, Helliwell TR, Kinsella A, Jones AS. P53 expression and p53 gene mutation in oral cancer and dysplasia.Otolaryngol Head Neck Surg 1998; 118: 115-23. [Pubmed]
5. Nylander K, Nilsson P, Mehle C, Roos G. P53 mutations, protein expression and cell proliferation in squamous cell carcinomas of the head and neck. Br J Cancer 1995; 71: 826 – 30. [Pubmed] [Full text]
6. Wood MW, Medina JE, Thompson GC, Houck JR, Min KW. Accumulation of the p53 tumor suppressor gene product in oral leukoplakia.Otolaryngol Head neck Surg 1994; 111: 758 – 63. [Pubmed]
7. Wynford – Thomas D. P53 in tumor pathology: can we trust immunocytochemistry? J Pathol 1992; 166: 329 – 30. [Pubmed]
8. Werness BA, Levine AJ, Howley PM. The E6 proteins encoded by human papilloma virus type 16 and 18 can complex p53 in vitro. Science 1990; 248: 76 – 9. [Pubmed]
9. Vered M, Allon I, Dayan D. Maspin, p53 and Ki-67 in epithelial lesions of the tongue: from hyperplasia to dysplasia to carcinoma. J Oral Pathol Med. 2009; 38(3): 314-20. [Pubmed]
10. Kumar P, Kane S, Rathod GP. Coexpression of p53 and Ki-67 and lack of c-erb B2 expression in oral leukoplakia in India.Braz Oral Res. 2012 May- June; 26(3): 228-34. [Pubmed] [Full text]
11. Ranganathan K, Kavitha R. Proliferation and apoptosis markers in oral submucous fibrosis. J Oral MaxillofacPathol. 2011; 15(2): 148-53. [Pubmed] [Full Text]
|