PART II - RECENT MATERIALS
Introduction:
There has been tremendous progress in Orthodontics since Edward Angle first popularized the fixed orthodontic appliance at the turn of the century. Recent years have seen an increased demand for orthodontic treatment from both adolescents and adults. In addition, patient and clinical expectations of treatment outcomes continue to rise. A desire for more aesthetic materials has resulted in both smaller and ‘tooth colored’ appliances[1]. The first part of paper has dealt with various advances in the field of diagnosis and treatment planning. The second part deals with various materialialistic advancements that have graced the profession of Orthodontics in recent years. These include:
1. Bonding agents:
Adhesive bonding is important for orthodontics, especially in terms of the fixation of bands and brackets to teeth. Dental cements and resins are used intraorally to secure fixed orthodontic devices. Although cements are still used, the popularity of resin and resin-cement hybrid materials is increasing because of their improved physical properties and low solubility in oral fluids. Bonding of orthodontic brackets to the tooth enamel has been an important issue since the introduction of direct bonding in orthodontics. Since then, many new bonding agents have been developed such as composite resins, conventional glass ionomer cements, resin-modified glass-ionomer cements and polyacid modified composites (compomers).
1. Composites:
2. Flouride releasing composites:
The fluoride within this material is not bound, but rather encapsulated within the composite, which allows the fluoride to be released by a diffusion/dissolution mechanism over a prolonged period to the adjacent enamel. Their advantages include that it is available as a light-activated composite and has a unique properly of fluorescing under ultraviolet light[2]. For eg: Transbond XT (Fig 1).
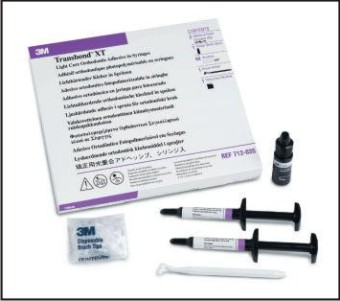 | Fig 1 : Fluoride Releasing Composite
 |
1. Moisture resistant adhesives:
Conventional acid-etching and priming adhesive system require dry conditions because of their hydrophobic properties. It is available in a primer formulation that replaces the conventional bonding agents. The main reactive component of this product is a methacrylate-functionalized polyalkenoic acid copolymer. Excess interfacial water ionizes carboxylic groups, forming hydrogen- bonded dimmers[3] (Fig 2).
 | Fig 2 : Moisture Resistant Adhesives
 |
1. Moisture active adhesives:
Moisture-active adhesives (Fig 3) require rather than tolerate the presence of moisture for proper polymerization. The surface must be intentionally wetted prior to application. These require no bonding agent. These are based on a cyanoacrylate formulation (Smartbond). These are useful in conditions where moisture control is difficult[3].
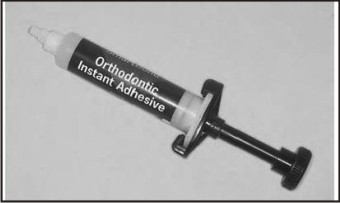 | Fig 3 : Moisture Active Adhesives
 |
1. Dentin Bonding agents:
2. Fluoride Releasing Bonding Agents:
Recently manufacturers have developed various fluoride releasing resin adhesives (Fig 4) because fluoride exerts anticariogence activity by increasing enamel and dentin resistance to subsequent acid attack. Fluoride is incorporated as silanized NaF porticles or pre-reacted glass particle fillers. It has been shown that fluoride incorporated into adhesive resins increased the dentin bond strength and it did not decrease after long term water immersion.
 | Fig 4 : Fluoride Releasing Bonding Agent
 |
In 1996, Saito demonstrated that the tensile bond strengths of methyl methacrylate resin containing fluoride did not decrease after 18 months of water immersion, while the bond strength of the some system without fluoride decreased during the same immersion time. Fluorides might reduce the solubility of intrinsic calcium phosphates within hybrid layer which otherwise would tend to solubilize in water overtimes, thus resulting in stable bond strength to dentin over time. Examples: Clearfil SE Bond Plus (Kuraray), One –up bond F (Tokuyama, Tapan), G-bond (GC America)[3].
1. Nano-filled adhesives:
These are generally amorphous silicon dioxides which are 100 times smaller than the fillers in hybrid composites that exhibit optimal optical properties. In 2004, Moszner and Klapdohr developed fillers with size ranging from approximately 5-100nm which increased adhesive strength to both enamel and dentin, increased marginal integrity, sufficient film thickness for one cost one cure technique and deeper penetration into dentinal tubules[4] (Fig 5).
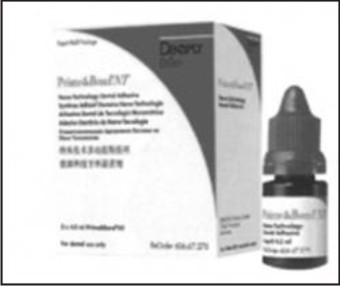 | Fig 5 : Nanofilled Adhesive
 |
1. Antibacterial self etching primer:
12- ethacryloyloxydodecylpyridinium bromide (MDPB) is an antibacterial agent incorporated into a self-etching adhesive system (Fig 6) to inhibit bacterial attachment and plaque accumulation on the tooth surface[5].
 | Fig 6 : Antibacterial Self Etching Primer
 |
1. Cements:
These were used very extensively before the advent of resin materials. Their use has seen a significant decrease in recent times. Yet, they are used routinely throughout the world for cementation of orthodontic bands and other appliances.
(a) Glass Ionomer Cements Containing Chlorhexidine and Cetrimide:
Several attempts in developing GIC with enhanced antibacterial effects by addition of bactericides, such as, chlorhexidine hydrochloride, cetyl pyridinium chloride, cetrimide, and benzalkonium chloride have been reported in the literature. The most appropriate choice of antibacterial agents to combine with GIC would be antiseptic agents that have proven to be useful in clinical dentistry and are the ones that do not disturb the physical properties. GICs containing chlorhexidinediacetate and cetrimide were effective in inhibiting bacteria associated with caries, and incorporation of 1% cetrimide was optimal to give the appropriate antibacterial and physical properties[6].
(b) Resin – ionomer hybrids:
Hybrid materials have been developed because traditional GICs have several disadvantages like short working time, long setting time, cracking on desiccation, poor resistance to acid attack, low fracture toughness, low abrasion resistance, initial sensitivity to moisture and higher bond failure rate (20%).
It was introduced by Antonucci in 1988 .The new generation of resin-modifiedglass ionomer cements, which include varying amounts 10%-20% of a photocurable monomer.The size of the glass particles may also be altered for the intended use of the material. Large particle sizes (50 µm) are used for the restorative cements, and smaller particle sizes (20 µm) are used for the luting cements. These set by an acid-base reaction and by free-radical addition polymerization (which may be light or chemically activated). They contain components present in both GICs and resin composites.
Conventional GICs have the disadvantage of moisturesensitivity and low early strength. Their drawbacks are covered up by formulations that impart additional curing process by addition of polymerizable functional groups. This gives improved lining and restorative materials with an immediate command set with higher early strength and water resistance. They can be activated by light or chemicals. This group of material is identified as light cured GIC (Fig 7), dual cured GIC (for light cured and acid-base reaction), tri-cured (dual cured, plus chemical cured)[7].
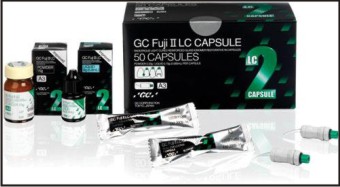 | Fig 7 : Light Cure Gic
 |
(c) Polyacid - modified composite resin or Compomer:
Compomers are single-component systems consisting of aluminosilicate glass in the presence of carboxyl-modified resin monomers andlight-activated conventional resin monomers. They were developedto bring the advantages of glass ionomer cements (fluoride release,chemical adhesion, or chelation) to resin based adhesives. Compomer (Fig 8) is a contraction of "composite and"lonomer," suggesting a material with intermediatecharacteristics and properties. Compomers arepackaged and handled much like composites. Theseare supplied as anhydrous single paste[8].
 | Fig 8 : Compomer
 |
Advantages:
1. They have excellent esthetics.
2. Low solubility
3. High bond strength
4. High fracture toughness
Drawbacks:
1. They require bonding agents to bond with the tooth structure.
2. They have generally been shown to have less fluoride release than glass ionomers.
3. Brackets:
4. Ceramic brackets:
Ceramic brackets (Fig 9) serve as a cosmetic alternative to traditional metal braces by blending in more with the natural color of the teeth. Ceramics are a broad class of materials that include precious stones, glasses, clays, mixtures of ceramic compounds, and metallic oxides. In essence, a ceramic is neither metallic nor polymeric. All currently available ceramic brackets are composed of aluminum oxide. However, because of their distinct differences during fabrication, there are two types of ceramic brackets, namely, polycrystalline alumina and the monocrystalline alumina. The manufacturing process plays a very important role in the clinical performance of the ceramic brackets[9],[10]. The nature of manufacture leaves behind surface roughness and micro cracks predisposing to increased friction and brittle fracture. The result is optically clear bracket with minimal impurities and imperfections[11].
In an attempt to improve the frictional characteristics of polycrystallineceramic brackets, Kusy and Whitley[12] suggested metal lined / reinforcedarchwire slots as the morphologies of metal inserts are improved, these metal-lined ceramic brackets (Fig 10) will provide not only good esthetics among ceramic brackets but also minimal friction among conventionally ligated brackets.
 | Fig 9 : Ceramic Brackets
 |
 | Fig 10 : Metal Lined Ceramic Brackets
 |
1. Plastic brackets:
Plastic braces (Fig 11) also serve as a cosmetic alternative to metal braces and have a less conspicuous or hidden appearance. In 1965 Newman introduced plastic brackets to improve the appearance during orthodontic treatment. The application of plastic brackets, however, has been limited because of their problems of durability and colour stability[13],[14].
 | Fig 11 : Plastic Brackets
 |
1. Titanium brackets:
Titanium brackets (Fig 12) resemble the stainless steel braces but are lighter and strong. They also provide an alternative to stainless steel brackets in patients who had sensitivity to nickel. The advantages of titanium brackets include excellent biocompatibility, small size, matte finish, low thermal conductivity and no metallic taste but they are more expensive than stainless steel[15].
 | Fig 12 : Titanium Brackets
 |
1. Self-ligating brackets:
Self-ligating brackets (Fig 13) utilizes a permanently installed, movable component to entrap the archwire. The most significant difference from the conventional bracket is that they don't need tie wires or elastic ligatures to hold the arch wire onto the bracket and hence are considered to be more aesthetic. They are smaller in size than the conventional brackets. They are held on by a "trap door" built into each bracket and use a slide mechanism to hold the archwire, thus reducing the amount of pressure exerted on teeth[16]. Advantages of self-ligating brackets include greater patient comfort, use of lighter forces, low friction, reduced risk of percutaneous injury, easy to clean, reduced chair time, more precise control of tooth translation, shorter treatment time[17].
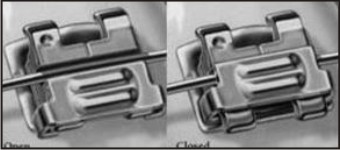 | Fig 13 : Self-ligating Brackets
 |
1. Lingual brackets:
Lingual treatment has advantages over labial treatment for the patients that it is not visible, provided the course of treatment and the quality of the results were the same as with a conventional treatment. Lingual brackets (Fig 14) thus represent the best solution for meeting the needs of patients without the risk of damaging biomechanical efficiency.One of the most significant drawbacks to lingual therapy appears to be the discomfort to the tongue and difficulty in speech. Also, the sensitivity of the laboratory techniques and the extended chair time needed for appliance placement and adjustments have made the treatment prohibitively expensive for many patients[18].
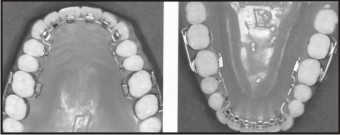 | Fig 14 : Lingual Brackets
 |
1. Wires:
2. Dual flex archwires:
The key to success in a multi attachment straight wire system is to have the ability to use light tipping movements in combination with rigid translation and to be able to vary the location of either, at any time the need arises during treatment.They used three specific combined wires for the technique; Dual Flex wires (Fig 15). These are of 3 types: Type1, type 2 and type 3.
 | Fig 15 : Dual Flex Archwire
 |
The Dual flex type 1 archhas its anterior segment made up of 0.016" titanol. It is a Nickel Titanium alloy manufactured by Lancer pacific, the posterior segment is made up of 0.016" Stainless Steel thus combining anterior and posterior segments of different stiffness.The flexibility of Titanol anterior segment greatly simplifies bracket engagement in the crowded anterior teeth while the rigidity of stainless steel posterior segment controls rotation movements, prevents tipping from elastic traction and permit bite opening. It is ideal for lingual appliance where anterior interbracket width is greatly reduced.
Dual flex type 2 arch wire is of 0.016" x 0.022" rectangular Titanol wires in the anterior and 0.018" Stainless Steel posterior segmental wire. It is useful in retraction of anterior teeth to upright position, where it does not need all of extraction spaces. Engagement of rectangular anterior titanol segment in the bracket slots impedes movement of the anterior teeth while closing remaining extraction space by mesial movement of posterior teeth.
The Dual Flex-2 and 3 wires establish anterior anchorage and control molar rotation during the closure of posterior spaces. They also initiate the anterior torque[19].
1. Teflon Coated archwires:
Teflon (Polytetrafluoro ethylene) was prepared by PUNKEIT in the United States in 1938.Teflon coated archwires (Fig 16) are available in natural tooth shades and in other colors such as blue, green, purple. The advantages claimed are esthetics and low friction between teflon and the bracket, which enhances sliding mechanics.Teflon coating imparts to the wire a hue which is similar to that of natural teeth. The coating is applied by an atomic process that forms a layer of about 20-25μm thickness on the wire. This layer then undergoes a heating process and acquires a surface with excellent sliding properties and substrate adhesion. Teflon coating protects the underlying wire from the corrosion process. However, since this coating is subject to flaws that may occur during clinical use, corrosion of the underlying wire is likely to take place after its prolonged use in the oral cavity[20].
 | Fig 16 : Teflon Coated Archwire
 |
1. Optiflex Wires:
Optiflex (Fig 17) is a recently introduced arch wire by Tallas. It combines highly aesthetic appearance with unique mechanical properties. It is made of clean optical fiber and consists of 3 layers.
 | Fig 17 : Optiflex Archwire
 |
A - Silicon-di-oxide core that provides the force for moving teeth.
B- Silicon resin middle layer that protects the core from moisture and adds strength.
C- Strain resistant nylon outer layer that prevents damage to the wire and further increases its strength (Fig 18).
The wire is manufactured in various sizes, and can be either round or rectangular. Sharp bends must be avoided, since they could fracture the core. It is highly resilient arch wire that is especially effective in the alignment of crowded teeth. It has got a wide range of action and apply light continuous force[21].
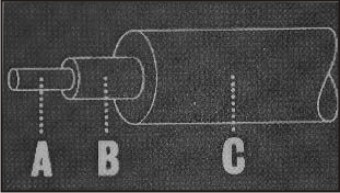 | Fig 18 : Structural Layers Of Optiflex Wire
 |
1. Dead Soft Security Arch Wires:
It has been introduced by Binder and Scott[22]. In a non-extraction case, an arch wire is usually placed to initiate tooth movement immediately after bonding. However, in an extraction case a proper arch wire might create undesired tooth movement before extractions are performed. This problem can be avoided by placing sectional arches made of dead soft brass wire or twisted double strands of 0.008″ or 0.010″ dead soft stainless steel ligature wires. These arches are bend to lie passively in all attachments. The same type of sectional arches can be used as final arch wires in one or both arches in conjugation with snake elastics to enhance intercuspation prior to appliance removal.
1. Bioforce wire:
GAC offers the BioForce (Fig 19) single-strand, superelastic, body-heat-activated archwire that starts with biologically correct, gentle forces for the anteriors, and then automatically increases in force at the posterior.The BioForce torques, levels, and aligns simultaneously. It features the IonGuard process, which makes nickel reactions virtually impossible and dramatically reduces friction and breakage[14].
 | Fig 19 : Bioforce Wire
 |
1. Composite wires:
A new generation material that incorporates long fibers such as glass or carbon has been developed that possess properties approaching those of metals. These wires can be shaped passively to the shape of the arch and when light cured harden to form a hard and stiff orthodontic component[23].
1. Titanium Niobium wires:
In 2000, Titanium Niobium (TMA 05) was introduced as a new finishing wire. According to the manufacturer’s product information, Titanium – Niobium is soft and easy to form. It has stiffness 20% lower than TMA and 70% lower than SS wires thus resulting in an increase in the formability of the wire. The load deflection rate is the same as TMA wires. These wires are ideal as finishing wires as they can be bent more easily and still are able to maintain the low force levels required. There is no leaching out of nickel from this alloy[24].
1. Timolium Titanium wires:
Titanium niobiumis an innovative archwire designed for precise tooth-tooth finishing. This wire has the advantage of not having the range of action of Titanium Molybdenum alloy (TMA) but has the stiffness of TMA. Timolium archwire has almost the same frictional resistance and half the stiffness of stainless steel making it an ideal choice for finishing, aligning as well as leveling and Torquing throughout all phases of treatment. Timolium possesses comparatively low stiffness, better strength and behaves as an intermediate between stainless steel and TMA and hence can be tried for almost all clinical situations. Low springback and high formability of titanium-niobium archwire allows creation of finishing bends and thus it can be used as finishing archwire[25].
1. Polyphenylene archwires:
Polyphenylene wires are smooth wires with consistent cross-sectional dimensions, high spring-back, and good ductility. Forces delivered were generally similar to typical beta-titanium and nickel-titanium wires of somewhat smaller cross sections. The polyphenylene wire did experience stress relaxation for up to 75 hours. The force magnitudes place polyphenylene wires in the category of an alignment or leveling wire. High formability allowed shape bending similar to that associated with stainless steel wires[26].
Conclusion:
Recent advances in material science and technology has resulted in an array of opening new vistas in the field of Orthodontics. Materials with widely diverging properties are on the market today and their usage has profound implications on the appliance mechanics. The knowledge of these innovations in diagnostic aids, appliances, wires, materials and technologies have dramatically improved our efficiency, case acceptance and profitability and will continue to do so in the near future.
References:
1. Cunningham SJ, Jones SP, Hodges SJ et al. Advances in orthodontics. Prim Dent Care 2002; 9(1): 5-8.
2. Sonis and Snell. Fluoride-releasing, visible light-activated bonding system. Am J Orthod Dentofacial Orthop 1989; 306 – 311.
3. Eliades G, Watts DC, Eliades T. Dental Hard Tissues and Bonding. Interfacial Phenomena and Related Properties. 1st ed. New York: Springer; 2005.
4. Moszner N, Klapdohr S. Nanotechnology for dental composites. International Journal of Nanotechnology 2004; 1: 130-156.
5. Kitasako Y, Senpuku H. Growth-Inhibitory Effect of Antibacterial Self-Etching Primer on Mutans Streptococci Obtained from Arrested Carious Lesions. J Esthet Restor Dent 16:176–184, 2004.
6. Deepalakshmi M. Evaluation of the antibacterial and physical properties of glass ionomer cements containing chlorhexidine and cetrimide: An in-vitro study. Indian J Dent Res 2010; 21(4): 552.
7. Compton AM, Meyers CE, Hondrum SO, Lorton L. Comparison of shear bond strength of glass ionomer cements of a light-cured glass ionomer and a chemically cured glass ionomer for use as an orthodontic bonding agent. Am J Orthod Dentofacial Orthop 1992; 101: 138-144.
8. Millett DT, McCluskey LA, McAuley F, Creanor SL, Newell J, Love J. A Comparative Clinical Trial of a Compomer and a Resin Adhesive for Orthodontic Bonding. Angle Orthod 2000; 70: 233-240.
9. Birnie D. Ceramic brackets. Br J Orthod 1990; 17:71-5.
10. Swartz ML.D Ceramic Brackets. J Clin Orthod. 1988; 22:82 – 88.
11. Jyothindra Kumar K.Recent advances in orthodontic materials. Pogonion: Manual of the First Indian Orthodontic Society Post Graduate Students Convention.
12. Kusy RP, Whitley JQ.Frictional Resistances of Metal-lined Ceramic Brackets Versus ConventionalStainless Steel Brackets and Development of 3-D Friction Maps. Angle Orthod 2001; 71: 364-374.
13. Reynolds IR.A review of direct orthodontic bonding. Br J Orthod 1975; 2:171-8.
14. Eliades T, Eliades G, Brantley WA. Orthodontics Materials Scientific and Clinical Aspects. 1st ed. Thieme; 2001.
15. Kusy RP, Whitley JQ, Ambrose WW, Newman JG. Evaluation of titanium brackets for orthodontic treatment: Part 1 the passive configuration. Am J Orthod Dentofacial Orthop1998; 114(5): 558-572.
16. Self–ligating brackets. Wikipedia, the free encyclopedia. http://www.en.wikipedia. org/wiki/self-ligating brackets. Accessed 10, 2012.
17. Marshall SD, Currier GF, Hatch NE, Huang GJ et al. Self-ligating bracket claims. Am J Orthod Dentofacial Orthop2010; 138(2): 128-131.
18. Creekmore T. Lingual Orthodontics - Its renaissance. Am J Orthod Dentofacial Orthop1989; 96(2): 120-137.
19. Cannon JL. Dual-flex archwires. J Clin Orthod1984; 18(9):648-649.
20. Kapila S, Sachdeva R. Mechanical properties and clinical applications of orthodontic wires. Am J Orthod Dentofacial Orthop1989; 96: 100-109.
21. Tallas M F. Optiflex Arch Wires – Treatment of a skeletal class III open bite. J Clin Ortho 1992; 26(4): 245-252.
22. Binder RE, Allison S. Dead Soft Security Archwires. J Clin Ortho 2001; 11: 682.
23. Zufall SW, Kusy RP. Sliding mechanics of coated composite wires and the development of an engineering model for binding. Angle Orthod 2000; 70(1):34-47.
24. Daltras M, Denes G, Melsen B. Titanium-niobium, a new finishing wire alloy. J Dent Res 2000; 3: 6-14.
25. Vijayalakshmi RD, Nagachandran KS, Kummi P, Jayakumar P.A comparative evaluation of metallurgical properties of stainless steel and TMA archwires with timolium and titanium niobium archwires – An in vitro study. Indian J Dent Res 2009; 20(4): 448-452.
26. Burstone CJ, Liebler SAH, Goldberg AJ. Polyphenylene polymers as esthetic orthodontic archwires. Am J Orthod Dentofacial Orthop2011; 139(4 Supplement): e 391- e 398.
|