INTRODUCTION
One of the most common reasons for the patients to seek dental care is discolored anterior teeth. The discolored, non-vital anterior tooth is a common aesthetic concern and can have a profound effect on patient’s self-esteem, interaction with others and employability [1]. Tooth discoloration varies in etiology, appearance, localization, severity, and adhesion to the tooth structure. It can be defined as being extrinsic, intrinsic or a combination of both on the basis of localization and etiology [2]. Tooth color is determined by combination of phenomenon associated with optical properties and light. Intrinsic color is determined by optical properties of the enamel and dentin and their interaction with light. Extrinsic color depends on material absorption on the enamel surface. Any change in enamel, dentin, or coronal pulp structure can cause a change of light transmitting properties of the tooth [3].
The correct diagnosis of the cause is of great importance as it has profound effect on treatment outcome. Causes for intrinsic local discoloration are pulp necrosis, intrapulpal hemorrhage, pulp tissue remnants after endodontic treatment,
endodontic materials, leakage of coronal filling materials and internal root resorption. Intrapulpal hemorrhage during pulp removal followed by inadequate debridement will cause blood components to combine with putrefying pulpal tissue to form iron. If there is improper coronal seal and bacterial penetration, then these products combine with hydrogen sulphates produced by bacteria to dark colored iron sulfates which discolor the tooth grey by penetrating deep into the dentinal tubule.
Such teeth are frequently compromised owing to previous trauma, caries, endodontic therapy and failed restorations. Destructive invasive treatment options are likely to weaken the residual structure of the tooth. This can reduce the prognosis and challenge the long-term viability of the tooth, thereby initiating further prosthetic predicaments [1]. Increasing numbers of patients do not want their teeth ‘cut down’ for crowns and are electing an alternative, conservative approach.
Bleaching of endodontically treated intact teeth presenting with chromatic alterations is a conservative alternative to a more invasive esthetic treatment such as placement of crowns or veneers. Furthermore, when metal free restorations are planned, bleaching of the prosthetic core can be useful in improving the final esthetic results. These materials do not
depend on light transmission characteristics but also on the color of the teeth that are being restored [3].
In the walking bleach technique the root filling should be completed first, and a cervical seal must be established. Sodium perborate is mixed with water/hydrogen peroxide/carbamide peroxide and placed in the pulp chamber. This treatment requires a bonded temporary filling or a bonded resin composite to seal the access cavity [2]. The
bleaching agent should be changed every 3-7 days. The thermo catalytic technique involves placement of a Superoxol in the pulp chamber followed by heat application. The Inside/ outside technique uses carbamide peroxide in the bleaching trays. Cervical root resorption is a possible consequence of internal bleaching and is more frequently observed in teeth treated with the thermo-catalytic procedure (9).The reason for this is contributed to leaching of hydroxyl ions from
the chamber to cervical dentin and cementum, creating low pH and initiating inflammation and resorption. This has been a major cause of avoidance of this very simple and economical procedure .If care is taken to provide proper cervical seal , not to use H2O2 for paste and use Ca(OH)2/Catalase in the chamber after bleaching to reverse pH/scavenge hydroxyl ions post bleach complications can be avoided. A case of post RCT discoloration of 11,12 teeth referred for crown preparation successfully treated by walking bleach method taking precautions to prevent post bleach complication is presented here.
REVIEW OF LITERATURE
Reports on bleaching of discolored nonvital teeth were first reported during the middle of 19th century advocating different chemical agents like chlorinated lime, oxalic acid, chlorine compounds and solutions, sodium peroxide, NaOCl, and Mixture of 25% H2O2 and 75% ether (pyrozone )[7,8]. Superoxol (30 % H2O2) has been mentioned by Abbot in 1918. Prinz in 1924 recommended using heated solutions of sodium perborate and superoxol for cleaning of pulp cavity. Some
authors proposed using light, heat or electric currents to accelerate bleaching reaction by activating the bleaching agents [9, 10]. has a dubious prognosis. Certain metallic ions (mercury, silver, copper, iodine) are extremely difficult to remove or alter by bleaching. Brown reported that trauma or necrosis induced discoloration can be successfully bleached in
about 95% of the cases, compared with lower percentage for teeth discolored as a result of medicaments or restorations [11].
A root filling does not adequately prevent diffusion of bleaching agents from the pulpal chamber to the apical foramen [12,13]. Hansenbayless and David indicated that a base is required to prevent radicular penetration of bleaching agents. Therefore, sealing the root filling with a base is essential, for which a variety of dental materials such as glass – ionomer cements, intermediate restorative materials (IRM), hydraulic filling materials (cavit, coltosol), resin composites,
photo-activated temporary resin material (fermit), zinc oxide – eugenol cement, and zinc phosphate cement have been suggested as an interim sealing agent during bleaching techniques [14]. The shape of the cervical barrier should be similar to the external anatomic landmarks, thus reproducing CEJ position and interproximal bone level. A flat barrier, level with the labial CEJ, leaves a large portion of the proximal dentinal tubules unprotected. Therefore, a barrier should be determined by probing the level of the epithelial attachment at the mesial, distal and labial aspects of the tooth. The intracoronal level of the barrier is placed 1mm incisal to the corresponding external probing depth of the attachment. With
this method the coronal outline of the attachment defines the internal pattern of the shape and location of the barrier [15]. However, the impact of the bleaching agent on the discolored dentin should not be hampered by the cervical seal.
Dentin tubules at the coronal third of the root run in an oblique direction from the apex to the crown, so that the tubules at the CEJ are originating more apically inside the root canal. If bleaching of a cervical region of the tooth is required, a stepwise reduction of the labial part of the seal and use of a mild bleaching agent are recommended [13].
Sodium perborate in the form of monohydrate, trihydrate, or tetrahydrate is used as hydrogen peroxide-releasing agent. It has been used since 1907 as oxidizer and bleaching agent, especially in detergent powders and bleaching agents. It is
stable when dry; however in the presence of acid, warm air or water it breaks down to form sodium metaborate, hydrogen peroxide, and nascent oxygen. It is safer and easier to control than conc. H2O2. The whitening efficacy with either water or hydrogen peroxide is not different, only the time taken is more with water [16]. Sodium perborate
(tetrahydrate) mixed with distilled water in a ratio of 2:1 (g/ml) is a suitable bleaching agent .In case of severe discoloration, 3% hydrogen peroxide can be applied in lieu of water. The bleaching agent can be applied with an amalgam carrier or plastic instrument and plugger and should be changed every 3-7 days [17].
The walking bleach technique requires a sound coronal seal around the access cavity after application of bleaching paste in the chamber. It can be achieved with GIC, resin composite or compomer to ensure its effectiveness and to avoid
leakage of bleaching agent into the oral cavity. In addition, a good seal also prevents recontamination of the dentin with
microorganisms and reduces the risk of renewed staining [2]. Occurrence of external cervical resorption is a serious complication after internal bleaching procedures. Heithersay analyzed cervical resorption cases and reported 24.1% were caused by orthodontic treatment, 15.1% by dental trauma 5.1% by surgery and 3.9% by intracoronal bleaching. The combination of bleaching and history of trauma is the most important predisposing factor for cervical resorption [18].
There is a speculation that hydrogen peroxide can diffuse through the dentinal tubules, cementum and periodontal ligament and can reach the bone. Lado et al [19] suggested that application of bleaching agents lead to denaturation of dentin proteins by oxidizing agents as well as acidic pH which induces a foreign body reaction. Root canal dressings consisting of calcium hydroxide are able to induce a higher pH in dentin [20]. A calcium hydroxide dressing should be placed in the pulp cavity for buffering the acid ph that can occur on cervical roots surfaces after intracoronal application of bleaching agents [21].
Application of catalase for three minutes following intracoronal bleaching eliminates residual hydroxyl ions thus can prevent cervical resorption [4]. Acidulated thio urea is also an effective scavenger of residual hydrogen peroxide and hydroxyl radicals generated during intracoronal bleaching [6]. Thus thio urea can be incorporated in the bleaching protocol to avoid cervical resorption. The adhesive strength of resin composites and glass-ionomer cements to bleached enamel and dentin is temporarily reduced as reported by Swift et al in 1998[22].But Accari et al in 2007 conducted an Invitro study to measure the microtensile bond strength of a nanofilled composite resin to human
dentin after nonvital bleaching at different postbleaching time intervals. It was concluded that the definitive restoration can be accomplished immediately after nonvital bleaching treatment [23]. The calcium hydroxide suspension temporarily
placed into the pulp chamber after completion of the bleaching procedure does not interfere with adhesion of composite materials used for final restoration of the access cavity [24]. A postoperative radiograph after bleaching and regular follow-up radiographs are recommended to diagnose possible cervical resorption.[25]. Cervical resorption can be treated with a direct restoration after gaining surgical access to the defect at the earliest. It is recommendedto place
90% trichloroacetic acid on the affected margin to necrotize granulation tissue before restoration[18] .
CASE REPORT
A female patient aged 23 years was referred to Department of Conservative Dentistry and Endodontics with discolored front teeth. She gave history of trauma 13 years back, which did not cause much problem but for the chipping of a
tooth. 5 years after the trauma, she developed acute pain in front two teeth on right side. She went to a dentist and was treated by root canal treatment of these teeth. After RCT she started with orthodontic treatment and noticed slight change
in color of root canal treated teeth. The discoloration increased gradually. She reported after completion of her orthodontic treatment and wanted crowns on 11, 12 to improve her appearance. On examination she had severely
discolored 11, 12 teeth. Coronal seal was missing in 11. IOPA revealed a defective, poorly condensed and short root canal obturations.No cervical erosion was seen. Crown structure of 11 &12 was intact but for incisal chipping of 12.
Retreatment of 11and 12 teeth, followed by walking bleach in both teeth and then incisal buildup of 12 was planned. It was explained to her that if bleaching is not effective then only the veneers/crowns will be planned. She consented
for this.
Old gutta percha obturations were removed. There was lot of debris and pus drainage from the canal of 11 where coronal seal was missing. Cleaning and shaping of both 11&12 teeth followed by closed Ca (OH)2 dressing was given.
Obturation was completed after one week with post obturation coronal seal with IRM. After one week clinical photographs were taken with the shade guide (VITA 3-D). Even shade 5M 1 was lighter than the shade of discolored teeth. The access was re-established by removing IRM. Coronal gutta percha was also removed from canals about 2 mm apical to cervical line. All remnants of filling materials were removed in the chamber which was cleaned thoroughly with xylene and NaOCl. Apical barrier of 2 mm thickness of GIC was placed over root canal obturation in a shape conforming to the shape of outer CEJ and 1mm incisal to it by external probing of the CEJ and epithelial attachment.
Sodium perborate was mixed with distilled water in a ratio of 2:1.The paste was inserted in the chamber with plastic instrument and pressed with cotton. Enamel portion of lingual access was left clear for the coronal sealing with GIC.
Patient showed marked improvement in shade of 12 after one week. Tooth 11 was also lighter but cervical region was still darker. On removal of coronal seal, slight debris was seen inside the chamber of 11. On careful examination under
good intensity of light and using a hand lens, a vertical crack line was seen passing through centre of facial enamel of 11. Bonding agent (Prime and Bond NT) was applied in this region after etching the facial enamel. Again the sodium perborate paste was sealed in 11. In tooth 12, Ca(OH)2 paste was packed in chamber, since the shade improvement was satisfactory in it. After sealing the facial crack in 11 marked improvement in the shade was seen after 3 days only. Patient was recalled after one week. This time bleaching was satisfactory. So Ca(OH)2 paste was sealed for two
weeks in 11 also. After that both 11&12 were resealed with Composite and incisal buildup of 12 was done using Ceram X Duo kit. (Fig 1- 4).Patient was recalled every three months to see any appearance of cervical erosion which did not
happen till date i.e. one year after bleaching (fig 5). A follow up for another year is planned.
DISCUSSION
The bleaching of unsightly non vital discolored teeth has been documented for over 100 years. The bleaching agents used for root filled teeth are hydrogen peroxide, carbamide peroxide, and sodium perborate. Hydrogen peroxide is the active ingredient in currently used tooth bleaching materials. It can be applied directly or can be produced by chemical reaction from carbamide peroxide or sodium perborate. In thermo catalytic bleaching Superoxol is placed in the pulp chamber as well as on exterior and application of some heat source is used to fasten release of nascent oxygen. Cervical root resorption is a possible consequence of internal bleaching and is more frequently observed in teeth treatedwith the thermo-catalytic procedure (26). Application of heat to activate Superoxol leads to more incidences of post bleach external cervical erosion and is not recommended. Inside outside bleaching at home using carbamide peroxide in bleaching gel is safe but there are chances of collection of debris and contamination in the open pulp chamber when
tray is not in mouth. In- office laser bleaching is a dentist controlled bleaching and safe but may cost the patient quite a bit and one needs to buy specific equipments for the same. Walking bleach procedure, on the other hand can be undertaken in remotest of places also as no special equipment is required and material cost is also minimal. It is
safe if precautions are taken to apply proper cervical barrier conforming to outer CEJ and 1 mm incisal to it. A coronal seal with GIC or other bonded restoration for proper sealing of bleach (Sodium perborate + water/ carbamide peroxide)
in the chamber will ensure proper bleaching within 2-4 weeks. In teeth discolored after trauma, enamel crack lines should be carefully looked for before starting bleaching in these teeth, as this need to be blocked otherwise bleaching may not be effective. Further if catalase is available then it can be applied in the chamber for 3-5 minutes to
remove remaining hydroxyl ions or else calcium hydroxide can be sealed in the chamber for 2 weeks for reversal of pH to avoid complications, Acidulated thio-urea is also reported to scavenge remnants of hydroxyl ions thus including it in the
bleaching protocol can also remove hydroxyl ions to avoid possible complications.
CONCLUSION
It can be concluded that bleaching is an important and valuable adjunct in endodontic treatment to get good aesthetic results. Proper diagnosis, selection of bleaching materials,(Sodium perborate + water/ carbamide peroxide) in the chamber will ensure proper bleaching within 2-4 weeks. In teeth discolored after trauma, enamel crack lines should be carefully looked for before starting bleaching in these teeth, as this need to be blocked otherwise bleaching may not be
effective. Further if catalase is available then it can be applied in the chamber for 3-5 minutes to remove remaining hydroxyl ions or else calcium hydroxide can be sealed in the chamber for 2 weeks for reversal of pH to avoid complications, Acidulated thio-urea is also reported to scavenge remnants of hydroxyl ions thus including it in the bleaching protocol can also remove hydroxyl ions to avoid possible complications.
CONCLUSION
It can be concluded that bleaching is an important and valuable adjunct in endodontic treatment to get good aesthetic results. Proper diagnosis, selection of bleaching materials, placement technique, and an understanding of the biologic interaction with soft and hard tissues are all factors that determine not only immediate success but also long term success, safety, and patient satisfaction as well .One has to carefully look for any crack lines in enamel of teeth with
discoloration after trauma and seal it before bleaching for better results. Thus walking bleach once discarded because of possible external resorption, if performed carefully is still a very relevant conservative and cost effective treatment
alternative.
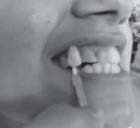 | Fig 1: Pre-operative photograph. Even M5 1 shade is lighter than stained teeth
 |
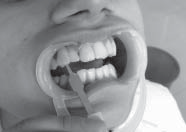 | Fig 2: Color change after one week with Na Perborate and water. Cervical third of 11 still needs improvement.
 |
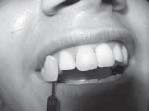 | Fig 3: Color improvement after 3days of sealing the vertical crack line on facial enamel with dentine bonding agent
 |
 | Fig 4: Final result with incisal buildup of 12 and sealing of access of 11 and 12 with composite.
 |
 | Fig 5. IOPA radiograph taken after one year
 |
REFERENCES
1. Poyser NJ; Kelleher MG; Briggs PF. Managing discoloured non-vital teeth: the inside/outside bleaching technique; Dent Update. 2004; 31(4):204-10, 213-4 (ISSN: 0305-5000)
2. Plotino G, Buono L, Grande NM, Pameijer CH, Somma F. Nonvital tooth bleaching: a review of the literature and clinical
procedures: J Endod. 2008 Apr; 34(4):394-407.
3. Joiner A. Tooth color; A review of literature. J Dent 2004;32: 3-12
4. Rotstein I. Role of catalase in the elimination of residual hydrogen peroxide following tooth bleaching. J Endod. 1993
Nov; 19(11):567-9.
5. Hara AT, Pimenta LA: Nonvital tooth bleaching. a 2-year case report. Quintessence Int. 1999; 30(11):748-54 (ISSN: 0033- 6572)
6. Farmer DS; Burcham P; Marin PD. The ability of thiourea toscavenge hydrogen peroxide and hydroxyl radicals during theintra-coronal bleaching of bloodstained root-filled teeth: AustDent J. 2006; 51(2):146-52 (ISSN: 0045-0421)
7. Truman J.Bleaching of nonvital discolored anterior teeth. Dent Times 1864; 69-72
8. Taft J. Bleaching teeth.Am J Dent Sci 1878/1879;12:364
9. Abbot CH. Bleaching discolored teeth by means of 30 % perhydrol and the electric light rays. J Allied Dent Society
1918;13:259
10. Prinz H. Recent improvement in tooth bleaching. A clinical syllabus. Dental cosmos1924;66: 558-560
11. Brown G. Factors influencing successful bleaching of discolored root filled teeth. Oral Surg Oral Med Oral Pathol
1965; 20: 238-244
12. Costas FL,Wong M . Intracoronal isolating barriers: effect of location on root canal leakage and effectiveness of bleaching agents. J Endod 1991;17:365-68
13. Smith JJ, Cunningham CJ, Montogomery S. Cervical canal leakage after internal bleaching procedures. J Endod 1992;18:476-81
14. Hansen-Bayless J, Davis R. Sealing ability of two intermediate restorative materials in bleached teeth. Am J Dent 1992; 151-4
15. Steiner DR, West JD. A method to determine the location andshape of an intracoronal bleach barrier. J Endod 1994;20:304-6
16. Ari H, Ungor M. Invitro comparison of different types of sodium perborate used for intracanal bleaching of discolored
teeth. Int Endodontic J 2002; 35: 433-436
17. Holmstrupg,Palm AM, Lambjerg- Hansen H. Bleaching of discolored root filled teeth. Endod Dent Traumatol 1988;4:
197-201
18. Heithersay GS:Invasive canal cervical resorption:Analysis of potential predisposing factors. Quintessence Int 1999;30: 83-95
19. Lado EA, Stanley HR, Weisman MI. Cervical resorption in bleached teeth. Oral Surg Oral Med Oral Pathol. 1983;
55(1):78-80.
20. Tronstad I,Andereasen JO, Hasselgren G, kristerson I, Reis I. pH changes in dental tissue after root canal filling with
Calcium Hydroxide. J Endod 1981;7: 17-21
21. Barateiri LN,Ritter AV,Monteiro S Jr ,Caldiera deAndrada MA,Cordoso Vieira LC. Nonvital tooth bleaching:guidelines
for clinician.Quntessence Int 1995;26:597-598
22. Swift EJ Jr,Perdigao J . Effect of bleaching on teeth and restoration. Compend Contin Educ Dent 1998;19:815-20
23. Arcari GM, Araújo E, Baratieri LN, Lopes GC. Micro tensile bond strength of a nanofilled composite resin to human dentin after nonvital tooth bleaching, J Adhes Dent. 2007; 9(3):333- 40 (ISSN: 1461-5185)
24. Demarco FF, Freitas JM, Siva MP, Justino LM. Microleakage in Endodontically treated teeth;Influence of calcium hydroxide dressing following bleaching.Int Endod J 2001;34:495-500
25. Amato M, Scaravilli MS, Farella M; Riccitiello F. Bleaching teeth treated endodontically: long-term evaluation of a case
series; J Endod. 2006; 32(4):376-378 (ISSN: 0099-2399) |