Introduction
The peripheral giant cell granuloma (PGCG) is a relatively common tumor-like growth of the oral cavity. It is an exophytic lesion of the oral cavity, also known as giant cell epulis, osteoclastoma, giant cell reparative granuloma, giant-cell hyperplasia, or peripheral giant cell reparative granuloma.[1] It is a benign hyperplastic lesion caused by local or chronic trauma. It originates from the connective tissue of the periodontal ligament or mucoperiosteum.[2],[3] Even though it is a common giant cell lesion found in the oral cavity,[4] it is the least commonly diagnosed lesion among the various hyperplastic gingival lesions e.g. pyogenic granuloma, fibrous hyperplasia and peripheral ossifying fibroma.[4],[5]
Chronic trauma can induce inflammation, produce granulation tissue with endothelial cells, chronic inflammatory cells and fibroblasts proliferation and manifests as an overgrowth called reactive hyperplasia. [6] PGCG is one of the most frequent reactive hyperplastic lesions of the jaws. These tumor-like lesions are not neo-plastic, but they indicate a chronic process in which an exaggerated repair occurs (granulation tissue and formation of scars) following injury.[3],[7],[8]
Clinical appearance of PGCG can present as polyploidy or nodular lesion, Primarily bluish red with a smooth shiny or mamillated surface stalky or sessile base, small and well demarcated. Pain is rare and in most cases the lesion is induced by constant trauma.[9] PGCG occurs exclusively on gingiva or edentulous alveolar ridge1 as variable sized, sessile or pedunculated lesion which is usually bleed easily.[10] They vary in size, though are rarely reported to exceed 2 cm in diameter.[11] However, there have been reports of masses in excess of 5 cm, where factors such as deficient oral hygiene or xerostomia appear to play an important role in lesion growth.[2] Incipient lesions induce minor changes in gingival contour but large ones adversely affect normal oral function.2 In some cases the underlying bone, suffers erosion and cup-shaped radiolucency occurs.[6] The lesion can develop at any age. It is, however, more common in the fifth and sixth decades of life with a slight female predilection. [3],[12]
The radiographic findings of PGCG are generally unremarkable, although tooth or osseous resorption may rarely be found in an adjacent area. Histologically, PGCGs present with giant cells in a vascular stroma of collagen fibers and are covered with stratified squamous epithelium. There are a large number of multinucleated giant cells resembling foreign body giant cells.[13],[14] It also appears as a non-capsulated mass of tissue containing a large number of young connective tissue cells and multinucleated giant cells.[15] Hem¬orrhage, hemosiderin, inflammatory cells, and newly formed bone or calcified material may also be seen throughout the cellular connective tis-sue.[4],[9],[16]
Treatment consists of local surgical excision down to the underlying bone,[1] for extensive clearing of the base.[10] Removal of local factors or irritants is also required.[17] If resection is only superficial, the growth may recur.[5] Exposure of all bony walls following thorough surgical resection responds satisfactorily most of the time.[12] Recurrence rate of 5.0-70.6% (average 9.9%) has been reported in various epidemiologic studies.[18] A recurrence rate of 5% has been reported by Giansanti and Waldron (1969)[19] while a study by Eversole and Rovin(1972)[20] showed a recurrence of 11%. Recurrences are believed to be related to lack of inclusion of the periosteum or periodontal ligament in the excised specimen.[17] A re-excision must be performed for these cases.[1] Aggressive tendencies[21] or malignant transformation of these lesions has never been reported.[4] PGCG lesions are self-limiting.[21]
Case Report
A 55 years old female patient who complained about gingival enlargement and pain while chewing was referred to our clinic. Her intraoral examination revealed a raised, round; sessile, smooth-edged mass 3 x 2.5 cm in diameter located on the right mandibular alveolar ridge and had no ulcerated surface (Figure-1). The patient's oral hygiene was poor and she was mostly edentulous, with a few teeth remaining. There was accumulation of plaque and calculus on those remaining teeth. She was systemically healthy and was not taking any medication. Radiological examination revealed no evidence of bony involvement (Figure-2). Excisional biopsy of the lesion was performed, followed by curettage of the underlying bone. Biopsy specimen was preserved in 10% formalin and sent to department of pathology (Figure-3). Routine histological examination with haematoxylin and eosin stain was performed. The specimen showed fibrovascular connective tissue stroma comprising of large number of proliferating plump fibroblasts having round to ovoid nuclei and numerous multinucleated giant cells of uniform size having nuclei up to 20 in number. Numerous blood vessels and foci of haemorrhage were evident, particularly around the periphery of the lesion. Areas of ossification and chronic inflammatory cell infiltrate, predominantly lymphocytes, were seen. The overlying epithelium was parakeratinized stratified squamous epithelium with irregular rete ridges, and showed areas of discontinuation. A large number of stromal fibroblastic cells and multinucleated giant cells were seen in 10X and 40X magnifications (Figure-4, Figure-5). The microscopic features of the lesion were consistent with PGCG. Postoperative healing was uneventful. No recurrence of the lesion was found 12 months after surgery (Figure-6).
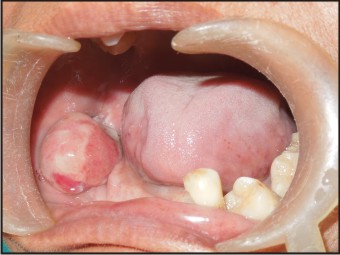 | Figure-1: Pre-operative View Of The Lesion
 |
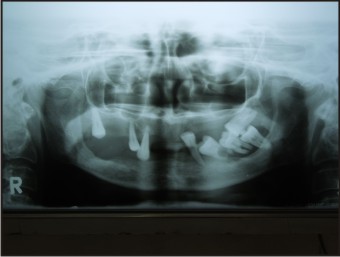 | Figure-2: Opg Radiograph
 |
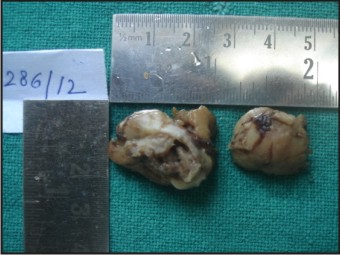 | Figure-3: Excised Specimen
 |
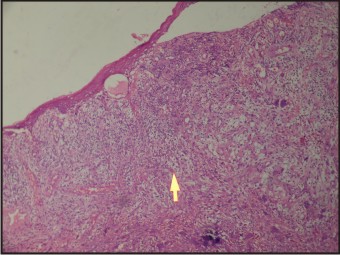 | Figure-4: Histopathology Of The Lesion (Marker Showing Giant Cell At 10x Magnification)
 |
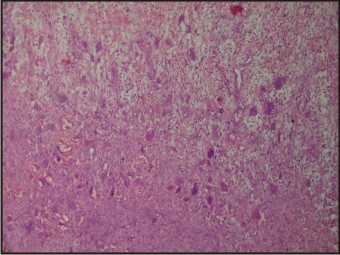 | Figure-5: Histopathology Of The Lesion (40x Magnification)
 |
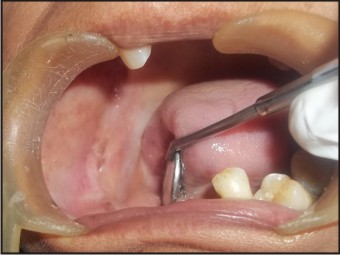 | Figure-6: Post-operative View Of The Site At 12 Months.
 |
Discussion
Jaffe first suggested the term “giant cell reparative granuloma” for the similar central lesion of the jaw bones[4] to help differentiate them from the giant cell tumor[12] as he believed the former lesion to represent a local reparative reaction rather than being a true neoplasm.[22],[23] Bernier and Cahn proposed the term“peripheral giant cell reparative granuloma” for the lesion.[4] The latter terminology is currently not being used as the reparative nature of the lesion has not been proved.[24] Today, the term peripheral giant cell granuloma is universally accepted.[4]
Many studies have been undertaken on the demographics and treatment modalities of PGCG. It has been reported in unusual places like mandibular condyle, nasopharynx, temporal bone and maxillary sinus. It has also been reported around dental implants (Table-1).
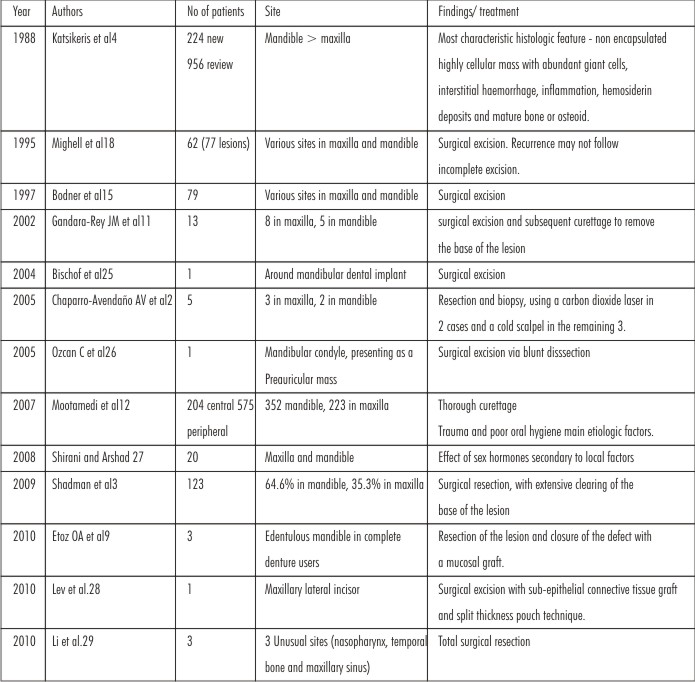 | Table : 1
 |
Li et al (2010)[29] investigated the clinicopathological features, diagnosis and treatment of giant cell granuloma outside the jaw. They retrospectively analyzed the clinical and pathological datas of 3 cases in nasopharynx, temporal bone and maxillary sinus, and also reviewed the relevant literatures. The patients in their study presented with local painless mass, part of which were aggressive. Histopathological feature was, replacement of the normal bone structure with proliferating fibrous tissue containing numerous giant cells was shown. The authors concluded that Giant cell granuloma outside the jaw was a non-neoplastic lesion, and extremely rare. It was somewhat difficult to make a correct diagnosis. Combining the clinical data and pathological feature were more helpful to the diagnosis. Total surgical resection was an effective therapeutic method.
Mighell et al (1995),[18] in their study, had identified the principle clinical features of the peripheral giant cell granuloma (PGCG), and recognised clinical features of PGCG that were poorly defined. They had reviewed retrospectively 77 cases of PGCG from 62 patients with respect to incidence, sex, patient age, race, clinical symptoms and signs, radiographic features and recurrence following excision.Their results was largely in agreement with previous reports. They have stated that there is wide variation in the results published between series. In addition, some clinical features of PGCG are poorly defined. Little is known about the relative incidences of PGCG and central giant cell granuloma. An association between PGCG and tooth loss may exist, but is poorly defined, and not all PGCG that involve edentulous areas follow recent tooth loss. Information about PGCG recurrence after excision is limited, and does not necessarily follow incomplete excision. Despite the large number of reported cases of PGCG, clarification of some clinical features is required, and may help formulation and interpretation of future laboratory-based research into this poorly understood lesion.
Motamedi et al (2007)[12] have assessed the demographic characteristics of PGCGs and central giant cell granulomas (CGCGs) in patients treated at their centers. Their 12-year retrospective study was based on existing data from 1993-2004. In their study, PGCGs presented in 575 patients, who varied in age from 2 to 85 years with a mean age of 31.02 years. Among these, 297 cases (51.65%) occurred in females and 278 (48.34%) in males. Four hundred sixty-seven cases (81.2%) occurred in the first five decades of life, and 352 cases (61.21%) appeared in the mandible. The authors concluded that PGCG lesions occurred more than 2 times more frequently than CGCGs. CGCGs occurred about 2 times more frequently in females, whereas PGCGs had an equal prevalence in both genders (P < .05). The mean age for patients with CGCGs was less than patients with PGCGs (P < .05). Central GCGs involved the mandible approximately 2 times more frequently than the maxilla (P < .05). However, when presenting in the maxilla, CGCGs most frequently presented in the area anterior to the canines (P < .05). Peripheral GCGs involved the mandible approximately 1.5 times more frequently than the maxilla (P < .05). Thorough curettage was the main treatment modality used. There were 9 cases (4.41%) of recurrence of CGCGs and 8 cases (1.39%) of recurrence of PGCGs documented during the follow-up period (ranging from 1-12 years).
PGCG is more common in the lower jaw rather than the upper jaw. [3], [12], [15], [19] The reported proportion is 2.4:1 and in most cases, it occurs anterior to molar region. [6],[16],[19],[30] However, according to Pindborg31 the preferential location is the premolar and the molar zones. According to Motamedi et al, PGCG more frequently involves the mandible, commonly in the areas posterior to canines.[12] PGCG affects females more than males, [4],[7],[16],[19] with a proportion of 1:1.5 or 1:2 according to Reichart and Philipsen [30] or Giansanti and Waldron studies[19] respectively. However Bhaskar et al,[21] Salum et al,[32] Zhang et al[33] and also Murat et al[34] reported a slight predilection for the male sex. But in some texts and studies, PGCG had an equal prevalence in both genders.[8],[12]
Giant cells may develop in normal bone or in pre-existing lesions, such as fibrous dysplasia, hyperparathyroid bone disease or, rarely, Paget’s disease.[35] The differential diagnosis of PGCG particularly involves giant cell tumor, nonossifying fibroma, pyogenic granuloma, CGCG, chondroblastoma and odontogenic cysts. [35],[36] Because GCG is indistinguishable from a brown tumor in microscopic examination, it must be ruled out from underlying hyperparathyroidism using laboratory and radiological methods. Malignant transformation has not been seen in any GCG cases.[36]
Timoscaand Gavrilită (1976) [37] have studied PGCG in a series of 173 patients amongst 894 with epulis type tumours. The highest occurrence rate of PGCG was found during the period of mixed dentition. Whilst in childhood PGCG was commoner in boys than girls, after the age of 16 the number of women affected was twice that of men. PGCG occurs more frequently in the mandible than the maxilla and more often in the premolar-molar region than the incisor-canine region. Osteolysis of the alveolar edge was more marked than in other forms of epulis and in a smaller number of cases osteogenic forms of PGCG were found with the formation of a substantial amount of reactive bone. From a histopathological standpoint, a connective-tissue/vascular stroma with giant cells and developing in relation with the capillary endothelium, and of similar appearance to CGCG was noted.
Dayan et al (1990)[38] have analysed Mineralized products in a series of 62 cases of peripheral giant cell granuloma of the gingiva or alveolar ridge of man. Histologic sections were examined by routine light microscopy and under polarized light to assess the extent and composition of mineralized tissues in these lesions. In 35% of the cases, mineralized tissue was identified in the form of woven and/or lamellar bone and dystrophic calcification. The most common type was the woven bone which appeared alone or in combination with lamellar bone in 82% of the lesions containing mineralized material. Unlike peripheral ossifying fibroma, no cementum-like material was identified in peripheral giant cell granuloma.
Bartel andPiatowska (1977)[39] have done an Electron microscopic study of peripheral giant-cell reparative granuloma. Six giant-cell tumors have been examined by electron microscopic methods in order to determine their histogenesis and to expand knowledge of cell structures. The examination of giant cells revealed an abundance of cell organelles, especially mitochondria. Apart from typical stromal cells, such as fibroblasts, macrophages, and mast cells, clusters of stromal "light" cells were often found with an intimate relation between their cell membranes. The role and function of these cells is not well known. Between fibroblasts of regular appearance with a small number of cytoplasmic organelles, others were found with an incrased number of mitochondria. These cells often formed aggregates. This suggests that giant cells develop through the association of stroma fibroblast cells.
The treatment of choice is surgical excision in cases of PGCG. The periosteum must be included in the excision to prevent recurrences. If resection is only superficial, the growth may recur. Most lesions respond satisfactorily to thorough surgical resection, with exposure of all the bone walls. When the periodontal membrane is affected, extraction of the adjacent teeth may prove necessary to insure full resection though this is initially contraindicated.[2],[40]
Curettage in addition to the excision to remove the base of the lesion also has been suggested. The recurrence rate of PGCG has been reported to range from 5–70.6%. This wide variation may be attributed to the surgical technique used in excision. The recurrence rate increases in hyperparathyroid cases. Long-term follow-up is mandatory because of the possibility of recurrence of the PGCG.[11],[41],[42] PGCG is a lesion of the gingiva mostly localized between the first molars and incisors.
Conclusion
In conclusion, the early and precise diagnosis of PGCG, based on the clinical and radiological findings and histological study, allows conservative management with a lesser risk for the teeth and adjacent bone. The characteristics and clinical behavior of PGCG may vary in different populations and be difficult to predict, reflecting different environmental influences, lifestyles, and racial factors, assessment of which may help in the diagnosis and management. Information regarding gender, age, signs, and symptoms may be useful and lead to an early diagnosis and proper management, preventing further damage to hard and soft tissues of involved areas. The usual line of treatment for PGCG is local excision down to the bony base along with elimination of the local etiological factors; failing to do so may result in the recurrence of the growth.
References
1. Neville BW, Damm DD, Allen CM, Bouquot JE. Soft Tissue Tumors. In Neville BW, Damm DD, Allen CM, Bouquot JE, eds. Oral and Maxillofacial Pathology 3rd ed. St. Louis:Saunders; 2009: 507-563.
2. Chaparro-Avendano AV, Berini-Aytes L, Gay-Escoda C. Peripheral giant cell granuloma. A report of five cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2005;10(1):53–7.
3. ShadmanN, EbrahimiSF, JafariS,EslamiM. Peripheral Giant Cell Granuloma: A Review of 123 Cases. Dent Res J (Isfahan). 2009 Spring; 6(1): 47–50
4. Katsikeris N, Kakarantza-Angelopoulou E, Angelopoulos AP. Peripheral giant cell granuloma. Clinicopathologic study of 224 new cases and review of 956 reported cases. Int J Oral Maxillofac Surg 1988; 17: 94-99.
5. Flaitz CM. Peripheral Giant Cell Granuloma: A potentially aggressive lesion in children. Pediatr Dent 2000; 22: 232.
6. Wood NK, Goaz PW. 5th ed. St.Louis: Mosby; 1997. Differential Diagnosis of Oral and Maxillofacial Lesions; pp. 141–2.
7. Carvalho YR, Loyola AM, Gomez RS, Araujo VC. Peripheral giant cell granuloma. An immunohisto-chemical and ultrastructural study. Oral Dis. 1995;1(1):20–5.
8. Regezi JA, Sciubba JJ, Jordan RCK. 5th ed. St.Louis: WB. Saunders; 2007. Oral Pathology: Clinical Pathologic Correlations; pp. 112–3.
9. Etoz OA, Demirbas AE, Bulbul M, Akay E. The Peripheralgiant cell granuloma in edentulous patients: report ofthree unique cases. Eu J Dent 2010; 4:329-33
10. Kfir Y, Buchner A, Hansen LS. Reactive lesions of the gingiva.A clinicopathological study of 741 cases. J Periodontol 1980;51: 655-61.
11. Gandara-Rey JM, Pacheco Martins Carneiro JL, Gandara-Vila P, Blanco-Carrion A, Garcia-Garcia A, Madrinan-Grana P, et al. Peripheral giant-cell granuloma. Review of 13 cases. Med Oral. 2002;7(4):254–9
12. Motamedi MH, Eshghyar N, Jafari SM, Lassemi E, Navi F, Abbas FM, et al. Peripheral and central giant cell granulomas of the jaws: a demographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(3):e39–e43.
13. Eronat N, Aktug M, Giinbay T, Unal T: Peripheralgiant cell granuloma: three case reports.J Clin Pediatr Dent 2000; 24: 245–248.
14. Özalp N, #0;ener E, Songur T. Peripheral Giant Cell Granuloma and Peripheral Ossifying Fibroma in Children: Two Case Reports. Med Princ Pract 2010;19:159–162
15. Bodner L, Peist M, Gatot A, Fliss DM:Growth potential of peripheral giant cellgranuloma. Oral Surg Oral Med Oral PatholOral Radiol Endod 1997; 83: 548–551.
16. Shafer WG, Hine MK, Levy BM. A textbook of oral pathol. 4th ed. Philadelphia: WB Saunders 1983:144-146.
17. Regezi JA, Sciubba JJ, Jordan RCK. Red-Blue lesions. InRegezi JA, Sciubba JJ, Jordan RCK, eds. Oral Pathology.Clinical Pathologic Correlations 5th ed. St. Louis: Saunders;2009: 107-25.
18. Mighell AJ, Robinson PA, Hume WJ. Peripheral giant cell granuloma: a clinical study of 77 cases from 62 patients, and literature review. Oral Dis. 1995 Mar;1(1):12-9.
19. Giansanti JS, Waldron CA. Peripheral giant cell granuloma: review of 720 cases. J Oral Surg 1969 Oct; 27: 787-91.
20. Eversole LR, Rovin S. Reactive lesions of gingiva. J OralPathol 1972; 1: 30-38.
21. Bhaskar SN, Cutright DE, Beasley JD, Perez B. Giant cell reparative granuloma (peripheral): report of 50 cases. J OralSurg 1971; 29: 110-15.
22. Kruse-L}5;sler B, Diallo R, Gaertner C, Mischke K-L, Joos U, Kleinheinz J. Central giant cell granuloma of the jaws: A clinical, radiologic and histopathologic study of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101: 346-54.
23. Jaffe HL. Giant cell reparative granuloma, traumatic bone cyst and fibrous (fibro-osseous) dysplasia of jaw bones. Oral Surg 1953; 6:159-75.
24. Carranza FA, Hogan EL. Gingival Enlargements. In Newman MG, Takei HH, Klokkevold PR, eds. Carranza’s ClinicalPeriodontology 10th ed. St Louis: Saunders; 2009: 373-390.
25. Bischof M, Nedir R, Lombardi T. Peripheral giant cell granuloma associated with dental implant. Int J Oral Maxillofac Implants 2004; 19(2): 295-299
26. Ozcan C, Apaydin FD, Görür K, Apa DD. Peripheral giant cell granuloma of the mandibular condyle presenting as a preauricular mass. Eur Arch Otorhinolaryngol.2005 Mar;262(3):178-81
27. Shirani G, Arshad M. Relationship between circulating levels of sex Hormones and peripheral giant cell granuloma. Acta Medica Iranica 2008; 46(5): 429-433.
28. Lev R, Moses O, Holtzclaw D, Tal H. Esthetic treatment of peripheral giant cell granuloma using a subepithelial connective tissue graft and a split-thickness pouch technique. J Periodontol.2010 Jul;81(7):1092-8.
29. Li S Wang S, Zhu L. Giant cell granuloma outside the jaw three cases and literature review. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.2010 Nov;24(22):1032-4.
30. Reichart P, Philipsen H P. Peripheral giant cell granuloma: review of 720 cases. J Oral Surg. 2000:164.
31. Pindborg JJ. 5th ed. London: Wiley-Blackwell; 1995. Atlas of Diseases of the Oral Mucosa; p. 186
32. Salum FG, Yurgel LS, Cherubini K, De Figueiredo MA, Medeiros IC, Nicola FS. Pyogenic granuloma, peripheral giant cell granuloma and peripheral ossifying fibroma: retrospective analysis of 138 cases. Minerva Stomatol. 2008;57(5):227–32.
33. Zhang W, Chen Y, An Z, Geng N, Bao D. Reactive gingival lesions: a retrospective study of 2, 439 cases. Quintessence Int. 2007;38(2):103–10.
34. Muratakgül H, Güngrmü M, Harorli A. Peripheral giant cell granuloma. a clinical and radiological study. 2004;16(1):59–63
35. Dorfman HD, Czernıak B (1998) Giant cell lesions. In: Bonetumors, 1st edn. Mosby, St. Louis, pp 559–605
36. Kaw YT (1994) Fine-needle aspiration cytology of central giantcell granuloma of the jaw. Acta Cytol 38:475–478
37. Timosca G, Gavrilită L.Peripheral giant cell granuloma of the jaw. Study of 173 casesRev Stomatol Chir Maxillofac.1976 Apr-May;77(3):587-97.
38. Dayan D, Buchner A, Spirer S. Bone formation in peripheral giant cell granuloma. J Periodontol.1990 Jul;61(7):444-6.
39. Bartel H, Piatowska D. Electron microscopic study of peripheral giant-cell reparative granuloma. Oral Surg Oral Med Oral Pathol.1977 Jan;43(1):82-96.
40. Warrington RD, Reese DJ, Allen G. The peripheral giant cell granuloma. Gen Dent. 1997;45(6):577–9
41. Breault LG, Fowler EB, Wolfang MJ, Lewis DW. Peripheral giant cell granuloma: a case report. Gen Dent 2000:716–719
42. Junquera LM, Lupi E, Lombardia E, Fresno MF. Multiple and syncronous peripheral giant cell granulomas ofthe gums. Ann Otol Rhinol Laryngol 2002;111:751–753
|