Introduction
The ongoing search for a biologically acceptable material that not only has physico-mechanical properties similar to those of natural tooth tissues, but is economical,substituting presumed toxicity of amalgam has dramatically increased.[1],[2] The expanding use of composite resin, particularly in relation to posterior restorations, is being limited despite constant improvements, presence of shrinkage from 2.6-7.1% during polymerization can cause post operative sensitivity due to rupture of adhesion between restoration and cavity wall. [3]
Several dentin bonding agents are being marketed, yet there are doubts concerning the longevity of the union because of hydrolysis of the resin leading to marginal leakage and secondary caries.[4] These contraction stresses are relieved by using materials with higher degree of elastic deformation during early stages of setting and hence glass ionomer cements have been recommended as base material.[5],[6]
In sandwich or double laminate technique the glass ionomer cement is used as an “underlay” to bonded resin composite which makes use of adhesive properties and biocompatibility of the glass ionomer cement and the desirable surface and esthetic appearance of the composite resin.[7],[8],[9]This technique is based on principle of ‘Biomimesis’ allowing the monolithic reconstruction of a tooth which is most valuable in conservative dentistry, minimizing some clinical problems related to microleakage and secondary caries.[10],[11]An important advancement in glass ionomer technology that has influenced dentistry is the introduction of resin-modified glass ionomer systems which seemed to overcome most of disadvantages of traditional glass ionomer which might be material of choice in laminate restorations due to their higher mechanical strength and less technique sensitive.[12],[13]
In original sandwich technique, after insertion of glass ionomer cement in the cavity, it is necessary to wait until the chemical cure or photoactivation of the material, and then can the acid etching ,rinsing, and drying be done followed by the application of the bonding agent and insertion of composite resin. The technique is too complex and long for children.[14],[15]
Dr G. Knight introduced the concept of co-curing when he accidentally cured specimens of light-activated glass ionomer and composite resin located together .This new technique eliminates the number of clinical steps as involved for Gold standard sandwich technique thus reducing technique sensitivity and increasing the efficacy of placement procedure.[16]
However, the usefulness of bonding is clear and the current concerns are centered around what materials, or combination of materials, best serves the needs of particular restorative problem.[17]Hence, this present study was conducted in vitro to evaluate and compare the shear bond strength at the glass ionomer and composite resin interface of this new technique with that of the gold standard sandwich technique using conventional and resin modified glass ionomer cement.
Subjects And Methods
The feasibility of bonding composite resin (Z100) to conventional glass ionomer (Fuji IX GP) and resin modified glass ionomer (VitremerTM) was evaluated by preparing fifty two specimens with two-part demountable Teflon mould .They were assigned into two groups of twenty six each as Fuji IX+Z100 and Vitremer +Z100 which were further subdivided into two subgroups as Set and Etched and Unset and Non-etched each consisting of thirteen samples.
In the Set and Etched subgroup of Fuji IX+Z100, the glass ionomer mix was placed in the 6mm´4mm cylindrical cavity drilled in the first Teflon mould, properly condensed, which was covered with glass microscope slide and static load of 500gms applied during its initial set of 7 minutes. The glass slide was carefully removed ensuring smooth glass ionomer surface was not pitted.
The exposed glass ionomer surface was acid etched with Scotchbond etchant (3M Products) for 15 seconds, washed, dried, followed by placement of Scotchbond Multipurpose primer then dried gently for 5 seconds. Scotchbond Multipurpose adhesive was applied on the primed surface using the applicator tip and light cured for 10 seconds. The second demountable Teflon mould with a cavity 4mm´4mm was centered on the first mold by slipping through the bolts, secured with help of tightening nuts to stabilize the specimens during its setting phase with screw tightened nuts to maintain assembly intact. Composite resin was added on top of the specimen, in two increments, thickness no greater than 2mm to ensure total light polymerization. Each increment compressed firmly, photo cured with TransluxÒlight held 1.0mm away from the resin surface for 40 seconds each, from two diametrically opposite directions.
The samples of Unset and Non-etched subgroup were prepared as similar to above subgroup but for the immediate application of Scotchbond Multipurpose primer on the unset and non-etched glass ionomer cement followed by adhesive applied using the applicator tip on the primed surface and light cured for 10 seconds prior to placement of composite resin with similar subsequent steps involved as above.
The bonding procedure for Set and Etched subgroup samples of Vitremer+Z100 group as the mixed glass ionomer cement was placed into the first Teflon mould with cavity of 6mm´4mm with same spatula, properly condensed; surface covered was covered with glass slide, light-activated by placing the wand of curing lamp directly applied against the glass for 40 seconds. The glass slide was carefully removed, the exposed glass ionomer surface was acid etched with etchant for 15 seconds,washed,dried,then primer was applied on the etched surface ,dried gently for 5 seconds. Subsequently using the applicator tip, Scotchbond Multipurpose adhesive was applied on the primed surface and light cured for 10 seconds. The second demountable Teflon mould with a cylindrical cavity of 4mm´4mm was centered on the first mould by slipping through the bolts, Composite resin was added on top of the specimen, in two increments, thickness no greater than 2mm to ensure total light polymerization. Each increment compressed firmly, photo cured for 40 seconds each with TransluxÒlight, from two diametrically opposite directions.
The samples preparation for Unset and Non-etched subgroup of Vitremer+Z100 group in that the mixed glass ionomer cement was placed into the first mould, condensed, but Vitremer cement was not photo-activated and no etching performed, primer was applied immediately with applicator tip, dried gently for 5 seconds followed by application of adhesive on the primed surface and light cured for 10 seconds. Afterward, composite resin was added on top of the specimen in two increments, photo cured for 40 second each, from two diametrically opposite directions as followed for above subgroup. The specimens were allowed to set for 30 minutes, and then the parts of jig were detached to remove bonded specimens. All bonded specimens were stored in distilled water for 24 hours at 370 C before shear bond strength testing. The glass ionomer component of the specimen was engaged into a specially designed guiding device with the composite resin side protruding from test assembly. The cutting edge (4mm) of the knife edge shearing chisel was then engaged at the glass ionomer –composite resin interface, force applied perpendicular to the long axis of specimen . The equipment was operated at cross head speed of 0.5mm/min and maximum load to debond the specimen was recorded in Newton (N).Shear bond strength was calculated in Mega Pascals (MPa) by the ratio of maximum load in Newton to the cross-sectional area of the bonded interface in mm.After debonding, the fracture site was carefully evaluated with Stereomicroscope-LEICA WILD M3Z at 40´magnification and were categorized as follows: Adhesion (A): Failure at the glass ionomer - composite resin interface, Cohesion(C): Complete failure within glass ionomer cement or composite resin, Mixed (M): Combination of Adhesion-cohesive failure.
Results
In FujiIX+Z100,the bond strength of Unset and Non-etched subgroup had higher value than the Set and Etched subgroup whereas in Vitremer+Z100 group the Set and Etched subgroup had mean values significantly higher than Unset and Non-etched subgroup with significant ‘t’ value (P<0.001)[Table 1].Intergroup comparison, the mean bond strength values were higher for Set and Etched subgroups ofVitremer+Z100group than compared to Set and Etched subgroups of Fuji IX+Z100group [Table 2]. Both One-way ANOVA and Two-way ANOVA revealed a significant difference in the mean shear strength values of different groups (P < 0.001) [Table 3,4]. The debonded specimens were examined using Stereomicroscope at 40x magnification revealed the failure types as shown [Table 5].
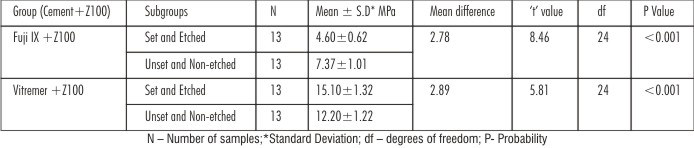 | Table 1: Intragroup comparison of shear bond strength using independent samples‘t’ test
 |
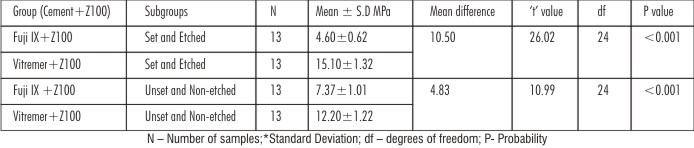 | Table 2: Intergroup comparison of shear bond strength using independent samples‘t’ test
 |
 | Table 3: Results of One-way ANOVA
 |
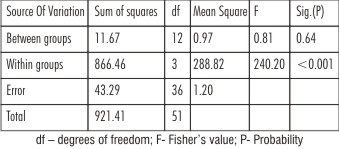 | Table 4: Results of Two-way ANOVA
 |
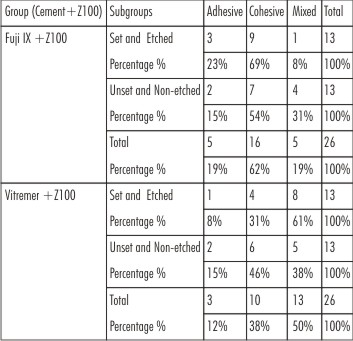 | Table 5: Percentage Distribution Of Failure Mode Of Fracture Sites In All Subgroups
 |
Discussion
The adhesion between glass-ionomer and the composite resin restricts the free surface area of shrinking composite, yielding higher polymerization stresses, which compete with the shrinkage vectors directed towards the light source. Because the adhesion between the etched glass-ionomer cement and the composite resin is stronger than the adhesion between the glass-ionomer cement and dentin, polymerization shrinkage of composite resin will “pull away” the glass ionomer cement from dentinal walls .[18]
The concept of co-curing is a new alternative for the union between glass ionomer and composite resin. In this, after insertion of glass ionomer cement into the cavity, the bonding agent is immediately applied and light cured prior to placement of resin where no need to wait for the setting of material or etch the ionomer surface, which appeared to enhance physical properties of the resulting restoration. Reduction in the number of operative steps, without a consequent decrease in the acceptability of clinical outcome, would help reduce the time of a dental appointment for the patient and the dentist.[14],[15],[16] Hence, this present study was conducted in vitro to evaluate and compare the shear bond strength at the glass ionomer and composite resin interface of this new technique, with that of the gold standard sandwich technique using conventional and resin modified glass ionomer cements.
Keeping in view various factors affecting the union of glass ionomer cement and composite resin like acid etching and etching times, cement strength, rate of set, effect of cement thickness, viscosity and wettability of bonding resin, materials with superior mechanical properties had been used .[19]
Fifty two cylindrical bonded specimens of each 10mmx4mm were prepared using a two-part demountable Teflon mould with aligning jig to confine the cement and resin. This 6mm´4mm specification for glass ionomer cement was in accordance to new ISO DIS 9917.[20],[21]
For Set and Etched subgroup, glass ionomer cement was allowed to initial set for 7 minutes, against glass microscopic slide to produce a smooth surface and static load was applied to compact the mass and reduce porosity.[22],[23]In Unset and Non-etched subgroup, the time lapse between the end of the mix and application of the bonding agent was maintained constant with the stopwatch, so that the bonding agent penetrates into the surface irregularities and hardens at the initial stages of setting of glass ionomer mass, resulting in mechanical attachment for better bond strength.[24]
In Fuji IX+Z100 group,the mean bond strength value of the Unset and Non-etched subgroup was significantly higher than Set and Etched subgroup mostly due to fact that low pH bonding agents etch the glass ionomer cement surface, and the matrix of glass ionomer mass dissolves, resulting in a rough and porous surface. The bonding agent penetrates into the surface irregularities and hardens resulting in mechanical attachment.The free phosphate phases may increase the polarity of the ionomer, while the monomer-bonded phosphate phases may bond primarily or secondarily to the glass substrate which preserves the external core of the ionomer at the critical stress-bearing interface.[23],[25]
In Vitremer +Z100 group,the Set and Etched subgroup had higher values than Unset and Non-etched subgroup probably the increased availability of unsaturated double bonds, in the air inhibited layer of resin-modified glass ionomer cements, may assist in chemical bonding to resin bonding agent and resin composite. Unpolymerized hydroxyethyl methacrylate, Unsaturated methacrylate pendants and modified polyacrylic acids on the surfaces increases the surface wetting capability of bonding agent and could increase bond strength when polymerized.[26]
The bond strength values of Unset and Non-etched subgroup were less than Set and Etched subgroup of Vitremer+Z100 group attributed to the omission of light activation shortly after the powder and liquid components are mixed, the mobility of polyalkeonate chains gradually decreased as they become more ionically cross-linked.[27] From the mean values it is clear that bond strength of Unset and Non-etched subgroup of Vitremer+Z100 group was higher than Unset and Non-etched subgroup of Fuji IX+Z100 group may due to fact that the resin modified glass ionomer bonded strongly to dental composite due to its similarity in chemistry providing a potential for chemical bonding between the materials.[28]
Majority of failure pattern in Set and Etched subgroup of Fuji IX+Z100 group are cohesive attributing the fact that acid etching of glass ionomer forms a weakened zone whichcan be partially reinforced with the bonding agent.[29]Set and Etched subgroup of Vitremer+Z100 group have also failed cohesively where all exhibited composite resin tags located at the center of the glass ionomer surfaces, thus indicating that the cohesive strength of resin modified cements is greatly increased compared with that of conventional cements.[26]
The fracture strength of brittle materials is strongly influenced by surface imperfections, which acts as stress concentrators. The nature of those surface defects is strongly influenced by the pretreatment. The unfilled resin may help offset the effect of the etch-induced surface flaws by wetting the glass-ionomer cement and filling asperities, minimizing their potential as crack nucleators. [29]
The properties of the tooth in concert with the restorative materials; under functional load determine the necessary level of bond strength. The conclusion of this in vitro investigation must be extrapolated to the in clinical situation with care, and further trials with these materials and surface treatments to confirm validity of these recommendations.
References
1. Liebenberg WH. Assuring restorative integrity in extensive posterior resin composite restorations: Pushing the envelope. Quintessence Int 2000; 31(3): 153-64.
2. Willems G, Lambrechts P, Braem M, Vanherle G. Composite resins in the 21st century. Quintessence Int 1993; 24 (9): 641-58.
3. Braga RR, Hilton TJ, Ferracane JL. Contraction stress of flowable composite materials and their efficacy as stress relieving layers. J Am Dent Assoc 2003; 134: 721-28.
4. Mount GJ. The tensile strength of the union between various glass ionomer cements and various composite resins. Aust Dent J 1989; 34 (2): 136-46.
5. Mc Lean JW. Dentinal bonding agents versus glass-ionomer cements. Quintessence Int 1996; 27: 659-67.
6. Burgess JO, Walker R, Davidson JM. Posterior resin-based composite: review of the literature. Pediatr Dent 2002; 24 (5): 465-79.
7. Croll TP, Nicholson JW. Glass ionomer cements in pediatric dentistry: review of the literature. Pediatr Dent 2002; 24 (5): 423-29.
8. Mount GJ, Hume WR. Preservation and restoration of tooth structure. London/Philadelphia/ St.Louis: Mosby International Ltd; 1998.
9. Mount GJ. Esthetics with glass-ionomer cements and the “sandwich” technique. Quintessence Int 1990; 21(2): 93-101.
10. Mount GJ, Ngo H .Minimal Intervention: A new concept for operative dentistry. Quintessence Int 2000; 31: 527-33.
11. Oilo G,Um CM. Bond strength of glass ionomer cement and composite resin combinations. Quintessence Int 1992; 23: 633-39.
12. Mc Lean JW. Evolution of Glass-Ionomer Cements: A Personal View. J Esthet Dent 1994; 6 (5):195-205.
13. Aboush YEY, Torabzadeh H. Clinical performance of class II restorations in which resin composite is laminated over resin-modified glass ionomer. Oper Dent 2000; 25: 367-73.
14. Pinheiro SL, Oda M, Matson E, Daurte DA, Guedes-Pinto ACG. Simultaneous Activation Technique: An alternative for bonding composite resin to glass ionomer. Pediatr Dent 2003; 25 (3): 270-74.
15. Milicich G. Auto-cure GIC-composite co-cure technique. Famdent 2003; 3: 15-19.
16. Knight GM. The co-cured, light-activated glass-ionomer cement-composite resin restoration. Quintessence Int 1994; 25 (2): 97-100.
17. Erickson RL, Glasspoole EA. Bonding to tooth structure: A comparison of glass-Ionomer and composite –resin systems. J Esthet Dent 1994; 6 (5): 227-42.
18. Meyers R, Gracia-Godoy F, Norling BK. Failure mode of posterior composite resin bonded to a glass ionomer cement treated with various etching times and with or without a coupling agent. Quintessence Int 1990; 21: 501-506.
19. Wilson AD, Mc Lean JW. Glass-Ionomer Cement. Chicago: Quintessence Publishing Co; 1988.
20. Nicholson JW, Mc Lean JW. A preliminary report on the effect of storage in water on the properties of commercial light-cured glass-ionomer cements. Br Dent J 1992; 173: 98-101.
21. Hinoura L, Moore K, Philips RW. Tensile bond strength between glass ionomer cements and composite resins. J Am Dent Assoc 1987; 114:167-72.
22. Garcia-Godoy F, Draheim RN, Titus HW. Shear bond strength of a posterior composite resin to glass ionomer bases. Quintessence Int 1988; 19 (5): 357-59.
23. Rao V, Reddy VV. An In vitro comparative evaluation of the tensile bond strength at the two interfaces of the sandwich technique. J Indian Soc Pedod Prev Dent 1995; 13: 10-12.
24. Hinoura K, Suzuki H, Onose H. Factors influencing bond strength between unetched glass ionomers and resins. Oper Dent 1991; 16: 90-95.
25. Papagiannoulis L, Eliades G, Lekka M. Etched glass ionomer liners: surface properties and interfacial profile with composite resins. J oral Rehab 1990; 17: 25-36.
26. Farah CS, Orton VG, Collard SM. Shear bond strength of chemical and light-cured glass ionomer cements bonded to resin composites. Aust Dent J 1998; 43(2): 81-86.
27. De Gee AJ, Leloup G, Werner A, Vreven J, Davidson CL. Structural integrity of resin-modified glass ionomers as affected by the delay or omission of light activation. J Dent Res 1998; 77 (8): 1658-63.
28. Li J, Liu Y, Liu Y, Soremark R, Sundstrom F. Flexural strength of resin-modified glass ionomer cements and their bond strength to dental composites. Acta Odontol Scand 1996; 54: 55-58.
29. Smith EDK, Martin FE. Acid etching of a glass ionomer base: SEM study. Aust Dent J 1990; 35 (3): 236-40.
|