Introduction
Tooth eruption is defined as the movement of the tooth from its site of development in alveolar bone to the occlusal plane in the oral cavity. The eruption process is complex and many different mechanisms are involved. It is divided into five stages: pre eruptive movements, intra-osseous stage, mucosal penetration, pre-occlusal and post occlusal stages.[1] A tooth or a group of teeth or entire dentition may be impacted at one of the above mentioned stages.
The eruption time of both primary and permanent teeth have got a specific date and variations of 6 months on either side of the usual eruption date may be considered normal for a given child. These days’ parents are worried about the variation in eruption timing, particularly delayed eruption of permanent teeth, which is considered as an important milestone in the growth and development of a child. Normally, the permanent teeth eruption into the oral cavity occurs at six years of age and continues up to thirteen years of age except third molars.
There are several factors which influence the eruption of permanent as well as primary teeth into the oral cavity that include local and systemic factors. In some cases, delayed tooth eruption, could be the first and foremost manifestation of local or systemic disease.[2] Marks and Cahill[3] stated that “tooth eruption is a series of metabolic events in alveolar bone characterized by bone resorption from occlusal surface of the developing tooth and bone formation takes place on opposite sides of the dental follicle and the tooth does not contribute to this process”. The factors that have related to the eruption of teeth include elongation of the root, forces exerted by the vascular tissues around and beneath the root, growth of alveolar bone, growth of dentin, growth and pull of periodontal ligament, nutritional and hormonal influences, pressure from muscular action and resorption of alveolar bone.[4] As the child grow, the metabolic demand of the tissues also increase, which might influence the eruption process as well. Aggarwal et al.[5] reported delayed eruption in deciduous dentition in malnourished Indian children. There is evidence that chronic malnutrition extending beyond the early childhood is correlated with delayed teeth eruption.[6] Delayed teeth eruption is also found to be one of the manifestation in syndromes like Cleidocranial dysplasia, Gardner syndrome, Down syndrome, Achondroplastic dwarF, Dysosteo sclerosis etc.[2]
Nutritional deficiency can affect endocrine glands of the body. Endocrine glands usually have profound effect on the entire body, including the dentition. Tooth eruption is influenced by pituitary growth hormone and thyroid hormone and parathyroid hormone related protein is required for tooth eruption.[7] Hence hypothyroidism, hypopituitarism, hypoparathyroidism and pseudo hypoparathyroidism are the most common endocrine disorders associated with delayed tooth eruption.[8] Vitamin D helps in the metabolism of calcium and phosphate which further are essential elements for skeletal and dental growth. Vitamin D also acts in concert with parathyroid hormone to maintain tight control of blood calcium concentration and stimulates secretion of parathyroid hormone, which mobilizes calcium and phosphorus from the skeleton to reestablish calcium homeostasis in the blood.[9]
Vitamin D refers to a group of fat-soluble secosteroids responsible for enhancing intestinal absorption of calcium, iron, magnesium, phosphate and zinc. In humans, the most important compounds in this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Cholecalciferol and ergocalciferol can be ingested from diet and from supplements. The body can also synthesize vitamin D especially cholecalciferol in the skin, from cholesterol, when sun exposure is adequate. Ultraviolet rays from the sun trigger vitamin D synthesis from its precursor, 7-dehydrocholesterol, in the skin.[10] In a country like India, where there is abundant sunlight throughout the year, vitamin D deficiency is unexpected.
Because vitamin D is an essential nutrient for proper skeletal development, children who receive too little can develop the bone disease like rickets.[11] Rickets is characterized by bone deformities, poor muscle development, spinal curvature and bowed legs.[12],[13] The presence of rickets during tooth development may result in enamel and dentin hypoplasia, incomplete development and delayed tooth eruption.[14]
Presented herewith is a case report wherein patient’s systemic deficiency of Vitamin D was diagnosed based on the clinical features of oral cavity (delayed teeth eruption).
Case Report
A patient of 10 years and 1 month old, belonging to a low-socioeconomic group family, presented with the chief complaint of not erupting permanent teeth. The child’s medical history was noncontributory. He was born with normal delivery and on proper time (such as in the ninth month of pregnancy). The patient’s height and weight was found to be matching with the chronological age (Fig 1). Extra-oral examination was also observed to be normal. On intra-oral examination, complete set of primary dentition was observed with caries in relation to maxillary and mandibular primary molars with generalized attrition (Fig 2, 3, 4). There was no mobility in any of the primary teeth present. The permanent first molars and mandibular permanent incisors were not erupted into the oral cavity, both of which should have been erupted at 6 years of age (Fig. 5). There were physiological spacing between incisors and primate spaces were present in canines, in both maxillary and mandibular arches.
 | Fig. 1: Extra-oral Photograph
 |
 | Fig: 2: Intra-oral Photograph
 |
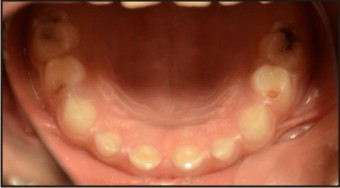 | Fig: 3: Intra-oral Maxillary Arch
 |
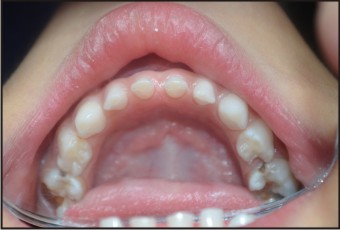 | Fig: 4: Intra-oral Mandibular Arch
 |
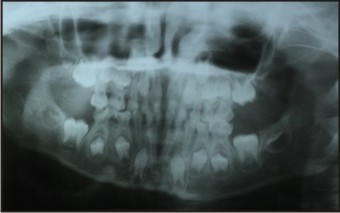 | Fig: 5: Orthopantomogram
 |
The dental age of the child seemed to be of less than six years since there is no permanent teeth present in the oral cavity. The chronological age of the child is more than10 years hence; there is delayed eruption of permanent teeth by 4-5 years. Clinically, there were no symptoms of child suffering from hypopituitarism or hypothyroidism or any other systemic disorder related to calcium and phosphate metabolism. The child was found to be receiving adequate sunlight exposure but there was history of inadequate intake of milk, green vegetable and required pulses.
The child was subjected to the following investigations of panaromic radiograph of jaws, serum level of T3, T4, TSH, PTH, Calcium, Phosphate and Vitamin-D. Radiographic investigation (Fig. 5) revealed the presence of all succedaneous teeth buds except mandibular lateral incisors at various levels of developmental stage. All the permanent first molars crowns were completed and initiation of root formation started. There were presence of crypts of developing permanent second molars seen in the OPG. No supernumerary or extra tooth buds or odontomes obstructing the eruption of permanent tooth or teeth were observed in the radiograph (Fig. 5). The radiographic evaluation confirmed the delayed eruption of permanent teeth.
Blood and hormonal investigations revealed severe deficiency of Vitamin-D (<12ng/ml), the normal ranges of Vitamin-D is considered to be in the range of 30.0 to 74.0 ng/ml. The levels of T3, T4, TSH and PTH were 120ng/dl, 5.2µg/dl, 20mg/l and 9.8mg/dl respectively. Other blood investigations were found to be within normal limits. Based on the clinical examination and investigations, final diagnosis of delayed eruption of teeth due to Vitamin-D deficiency was made.
Patient was prescribed oral Vitamin-D supplements 6,00,000 I.U weekly, in the form of powder which is to be taken with milk, for 1 month. Patient was also given instructions regarding good diet containing milk, milk products and green vegetables. He was reviewed after 1 month and again the blood investigation of 25-hydroxyvitamin D {25(OH)D} was done. The investigations revealed significant increase in the 25-hydroxyvitamin D levels (42 ng/ml) which comes well within the normal range.
Discussion
The eruption date of permanent maxillary and mandibular first molars are observed to be around 6-7 years, followed by mandibular central incisors 6-7 years, maxillary central incisors 7-8 years, mandibular lateral incisors 7-8 years, maxillary lateral incisors 8-9 years, mandibular canine 9-10 years, maxillary first premolar 10-11 years, mandibular first premolar 10-12 years, maxillary canine 11-12 years, maxillary second premolar 10-12 years, mandibular second premolar 11-12 years, mandibular second molar 11-13 years, maxillary second molar 12-13 years, and maxillary and mandibular third molars which erupt around 17-21 years of age.[15] Variation of six months on either side of eruption is considered to be normal and when there is excessive delayed of tooth eruptions are considered to be abnormal.
There is universal acceptance of eruption date of permanent teeth based on which we calculate the dental age of the child but there are some deviations of eruption time of permanent teeth observed in different ethnic groups in clinical practice.[1],[16] Pre-mature tooth eruption has also been reported in the literature,[17] but delayed tooth eruption is the most commonly encountered deviation from normal eruption time. By the term early or delayed tooth eruption, we mean entire dentition and not an individual tooth or a group of teeth. Delayed or early tooth eruption might be the primary or sole manifestation of systemic pathology.[18]
There are several systemic conditions which can lead to delayed tooth eruption.[19] These causes are related to pituitary, thyroid, parathyroid hormones and vitamin D deficiency or calcium, phosphate along with vitamin D deficiency.[18] There are few syndromes associated with the delayed eruption of teeth, which we discussed earlier. Hence diagnosis is an important but complicated process to establish the final diagnosis.
The local factors which are associated with premature or delayed eruption of teeth are also important to a pediatric dentist, to diagnose a tooth or a group of teeth which are erupting out of their time. The most common causes for delayed eruption or impaction of tooth or teeth are mesiodens and other supernumerary teeth,[20] ectopic eruption path, retained primary tooth, trauma, globulomaxillary cyst, odontomes, and lack of space in the dental arch.[21],[22] The most common cause for premature (early) eruption of tooth is caries associated with primary teeth, which causes destruction of bone above the growing tooth bud.[14] There is another factor such as dentigerous cyst (Odontogenic Cyst)[20] developed above the crown of a developing tooth bud and if the deciduous tooth is extracted for marsupialization, is seen with the early eruption of permanent tooth. The early eruptions of a tooth or group of teeth are utilized during the serial extraction plan cases which are not being discussed in this case.[1]
In the present case, there were no clinical symptoms of any systemic disorder observed which might help for the diagnosis. The delayed eruption of teeth led us to go for systemic investigations to find out the causes. Although data on nutrition (calcium, phosphate, vitamin D) influence on tooth eruption is scarce,[6],[23] there is evidence that chronic malnutrition extending beyond the early childhood is correlated with delayed teeth eruption.[24] Another important systemic factors which could cause delayed teeth eruption are hormonal (discussed previously) and or vitamin D deficiencies. Having reviewed all the systemic factors of delayed tooth eruption, emphasis was given on systemic investigations for the diagnosis in this particular case. Therefore, we investigated blood for T3, T4, TSH, serum calcium, phosphate and vitamin D levels. The result showed that there is deficiency of vitamin D (<12 ng/dl) in this particular case and all other factors were normal.
Vitamin D is important to bone mineralization because of its role in the maintenance of adequate serum calcium and phosphorus concentrations.[25] Vitamin D deficiency results in osteomalacia in adults or rickets in children and is a frequent source of occult muscle and bone pain.[26] There is increasing evidence that vitamin D can protect against the development of many chronic diseases, including type 1 diabetes mellitus, hypertension, cardiovascular disease, common cancers, multiple sclerosis and rheumatoid arthritis.[27] Rickets in infancy has also been reported to be associated with lower respiratory tract infections, the largest cause of infant mortality in India.[9] Other features of rickets could be craniotabes (areas of thickening and softening of bones of the skull), frontal bossing, delayed closure of anterior fontanelle, and weight bearing bones produces deformities such as bow-legs and knock-knees could be seen,[28] all of which were absent in this present case.
The condition presenting only vitamin D deficiency with the absence of any other systemic symptoms is otherwise called as “subclinical vitamin D deficiency”. In northern India, the vitamin D deficiency could be the result of taking solely vegetarian diet and not taking adequate fat containing diet inspite of good sunlight. Moreover, we do not have milk supply with added vitamin D supplements. This may lead to the deficiency of vitamin D in this particular patient, which might cause delayed eruption of teeth. Fatiha Zeghoud et al.[29] reported that subclinical low vitamin D status seldom causes neonatal rickets and had normalized serum PTH within 1 month only, when they were given vitamin D supplements.
After the proper diagnosis of vitamin D deficiency, the patient was later prescribed vitamin D supplements. He was prescribed vitamin D supplements in the form of sachets consisting 6 lakh units of vitamin D for 2months and was on follow up for 6 months, to which he responded well. On blood examinations, his vitamin D levels improved in subsequent follow-up visits following vitamin D therapy.
Conclusion
Delayed tooth eruption in a child is a worrisome experience for parents and it necessitates early investigations and interventions. There is rare literature or reports on delayed eruption of teeth due to vitamin D deficiency. This particular case is very interesting because delayed tooth eruption was the sole feature in this patient and without any other clinical symptoms of Vitamin D deficiency.
References
1. Profit. Contemporary orthodontics. 3rd ed. Mosby Inc.; 2000.
2. Shafer, Hine, Levy. Shafer’s Textbook of Oral Pathology. 6th edition. Elsevier Inc. 2009.
3. Cahill DR, Marks SC Jr, Wise GE, Gorski JP. A review and comparison of tooth eruption systems used in experimentation - a new proposal on tooth eruption. In: Davidovitch Z, editor. The biological mechanisms of tooth eruption and root resorption. Birmingham,AL: EBSCO Media; 1988. p. 1-7.
4. Wise GE, Frazier-Bowers S, D’Souza R.N.Cellular, molecular and genetic determinants of tooth eruption. Crit Rev Oral Biol Med 2002; 13: 323-34.
5. Aggarwal KN, Narula S, Faridi MM, Kalra N. Deciduous dentition and enamel defects. Indian Pediatr. 2003;40:124-9.
6. Psoter W, Gebrian B, Prophete S, Reid B, Katz R. Effect of early childhood malnutrition on tooth eruption in Haitian adolescents. Community Dent Oral Epidemiol 2008;36:179- 89.
7. Bedi R, Brook AH. Changes in general, craniofacial and dental development in juvenile hypothyroidism. Br Dent J. 1984; 157:58-60.
8. Loevy HT, Aduss H, Rosenthal IM. Tooth eruption and craniofacial development in congenital hypothyroidism: report of case. J Am Dent Assoc. 1987; 115:429-31.
9. Alok Sachan, Renu Gupta, Vinita Das, Anjoo Agarwal, Pradeep K Awasthi, and Vijayalakshmi Bhatia.High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr 2005; 81:1060–4.
10. Vin Tangpricha, Adrian Turner, Catherine Spina, Sheila Decastro, Tai C Chen, and Michael F Holick. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr 2004; 80:1645–9.
11. Preece M, McIntosh W, Tomlinson S. Ford I, Dunnigan M, O’Riordan I. Vitamin D deficiency among Asian immigrants to Britain. Lancet 1973; 1:907-10.
12. Pettifor IM, Isdale IM, Sahakian I, Hansen JDL. Diagnosis of subclinical rickets. Arch Dis Child 1980; 55:l55-7.
13. Ford JA, Davidson DC, Mc Intosh WB, Fyfe WM, Dunnigan MG. Neonatal rickets in Asian immigrant population. Br Med J. 1973; 3:21 1-2.
14. McDonald and Avery’s. Dentistry for the Child and Adolescent. 9th edition. Elsevier Mosby Inc; 2012.
15. Ash, Nelson. Wheeler’s Dental Anatomy, Physiology, and Occlusion. 8th edition. Elsevier Saunders Inc. 2003.
16. Nolla CM. The development of the human dentition. ASDC J Dent Child 1960; 27: 254-66.
17. Ekstrand KR, Christiansen J, Christiansen ME. Time and duration of eruption of first and second permanent molars: a longitudinal investigation. Community Dent Oral Epidemiol 2003; 31: 344-50.
18. Faizal C Peedikayil. Delayed Tooth Eruption- Review article. e-Journal of Dentistry. Oct - Dec 2011; 1(4): 81-86.
19. Ruta Almonaitiene, Irena Balciuniene, Janina Tutkuviene. Factors influencing permanent teeth eruption. Part one – general factors. Stomatologija, Baltic Dental and Maxillofacial Journal, 12: 67-72, 2010.
20. Suri, Gagari E, Vastardis H. Delayed tooth eruption:Pathogenesis,diagnosis and treatment. A literature review. Am J Dentofacial Ortho. 2004; 126;432-45.
21. Cunha RF, Boer FA, Torriani DD, Frossard WT.Natal and neonatal teeth: review of the literature. Pediatr Dent. 2001;23: 158-62.
22. Brin I, Ben-Bassat Y, Zilberman Y, Fuks A. Effect of trauma to the primary incisors on the alignment of their permanent successors in Israelis. Community Dent Oral Epidemiol 1988;16: 104-8.
23. Alvarez JO. Nutrition, tooth development, and dental caries. Am J Clin Nutr 2009; 61:410-6.
24. Balasubramanian K, Rajeswari J, Gulab,et al. Varying role of vitamin deficiency in the etiology of rickets in young children vs. adolescents in northern India. J Trop Pediatr. 2003; 49:201– 6.
25. Suda T, Ueno Y, Fujii K, Shinki T. Vitamin D and bone. J Cell Biochem. 2003;88(2):259–66.
26. Rajeswari J, Balasubramanian K, Bhatia V, Sharma VP, Agarwal AK.Aetiology and clinical profile of osteomalacia in adolescent girls in northern India. Natl Med J India. 2003; 16:139–42.
27. Brunvand L, Haga P, Tangsrud SE, Haug E. Congestive heart failure caused by vitamin D deficiency? Acta Paediatr. 1995:84:106-8. 21.
28. Gillore A, Groneck P. Kaiser I, Schmitz-Stolbrink A. Congestive heart failure in an infant due to vitamin D deficiency rickets. Monatsschr Kinderheilkd. 1989; 137: 108-110.
29. Fatiha Zeghoud, Christine Vervel, Huguette Guillozo, Odile Wairant-Debray, Henri Boutignon, and Michèle Garabédian.Subclinical vitamin D deficiency in neonates: definition and response to vitamin D. Am J Clin Nutr. 1997:65:771-8.
|