Introduction
Local anesthetics are the safest and most effective drugs employed for analgesia and pain management. Local anesthetic salts are water soluble and stable, existing simultaneously as cationic or charged (RNH+) molecules and deionized or uncharged (RN) molecules. The deionized free-base is referred to as the active form of the local anesthetic.[1] The active form of the local anesthetic is 4,000 times more lipid soluble than the cationic form, allowing easy penetration of the former into the nerve membrane.[2]
The pH of the solution determines the initial availability of the active form in the injection. The body’s mechanism for raising the pH of an acidic solution depends upon the bicarbonate present in the tissues at the injection site. The rate at which this buffering occurs depends upon the patient’s physiology and the condition of the tissues in the area of injection. At a physiologic pH of 7.4, the ratio of the active form to the cationic form is greatly improved to approximately one RN molecule for every three RNH+ molecules. The relative proportion of ionic forms also depends upon the dissociation constant (pKa) of the local anesthetic. Usually the infected tissues may have a pH as low as 5.0, which explains why infected teeth are more difficult or even impossible to numb.[1]
The pH of a solution is also a strong determinant of the latency or ‘onset time’. Raising the pH using sodium bicarbonate (NaHCO3) solution is an alternative to depending upon the body in accomplishing an equivalent pH change after the injection of local anesthesia. The ex vivo process (buffered LA) uses the same chemical mechanism and molecule (bicarbonate) as the body will use to buffer the anesthetic after the injection in vivo (inside the body), but the ex vivo process is an innovative way to accomplish the pH change instantaneously and more dependably.[1] The latency varies widely from patient to patient. Eliminating this variability and increasing predictability of onset, is a paramount feature of buffering anesthetic solutions outside the body.
The presentstudy compares in vivo and ex vivo alkalinization and evaluates the efficacy of sodium bicarbonate in creating a favorable environment for lignocaine to act around the inflamed and infected pulpal and periapical tissues of the mandibular teeth for painless exodontia.
Methodology
The study was conducted on patients reporting to the out-patient department of Oral & Maxillofacial Surgery, HIDS Paonta Sahib (HP), for the purpose of exodontia. 80 consenting patients aged between 15-72 years, who were divided into 2 groups of a sample size of 40 each. Group A received sodium bicarbonate infiltration for in vivo alkalinization and group B received buffered LA for ex vivo alkalinization. The study was independent of sex and weight of the patients and was conducted only upon the mandibular teeth. Teeth that were clinically tender on percussion were radiographically checked for the assessment of the periapical tissues.
In ‘group A’, the pain felt by the patient was scored using the visual analogue pain scale (VAS) with a 0-10 numeric pain rating scale.[2] The patients in group A underwent in vivo alkalinization with sodium bicarbonate infiltration. A standard ‘inferior alveolar nerve block’, along with ‘lingual nerve block’ was administered using a regular solution of lignocaine with adrenaline. A ‘long buccal nerve block’ was also given when the case involved the molar teeth. Approximately 2ml of local anesthetic solution was administered that contained 2% lignocaine with 1: 200,000 adrenaline. The ‘latency’ or the onset time of this regular LA containing adrenaline is noted. The subjective and objective signs and symptoms of local anesthesia were checked. A maximum of 10 minutes was allowed for the local anesthetic to take effect.
A chronically infected tooth that remained tender on percussion with anesthesia in the surrounding tissue, was made inclusive in this study group. In these cases of inadequate anesthesia, local infiltrations consisting of 0.5 ml 7.5% sodium bicarbonate solution were given in the buccal and lingual cortex at the position of the root apices of the tooth. The bone resorption caused due to chronic infection helped the bicarbonate solution to diffuse into the inflamed periapical tissues. We allowed a time of 3 minutes for this in vivo alkalinization to occur, which was the same as the maximum time taken by an infiltration of lignocaine with adrenaline to produce an analgesic effect. It raised the pH of the acidic tissues and created a favorable environment for the local anesthetic to work.
Local infiltrations each of 0.5 ml 2% lignocaine with adrenaline were then given buccally and lingually in the same areas. A solution was created in this region which contained both sodium bicarbonate and lignocaine with adrenaline in a 50-50 dilution. This led to the production of carbon dioxide which had its own independent anesthetic effect and acted as a catalyst to increase the potency of lignocaine.[1],[3] Pain was scored after 3 minutes using VAS. A reading of 3 or less on the scale was adequate for extraction.
In ‘group B’, the pain felt by the patient was scored using the VAS. The patients in group B underwent ex vivo alkalinization. 2 ml of ‘buffered LA’ is prepared with sodium bicarbonate and LA containing adrenaline used in a dilution of 1: 10.[4],[5] To every 10cc of 2% lignocaine with 1: 200,000 adrenaline, 1mEq of 7.5% sodium bicarbonate was added (i.e., 1.12 cc of 7.5%) to make a chair-side buffered LA solution in the syringe for injection. The latency of this buffered LA was noted. The pain was scored after the injection using VAS. A reading of 3 or less on the scale was adequate for extraction.
Teeth were extracted only after a score of 3 or less on the visual analogue pain scale. In case the VAS score was more than 3, the procedure was aborted and the extraction was done after 48 hours under oral antibiotic cover. Post-extraction instructions were verbally explained to all the patients.
Results And Discussion
Lignocaine is a weak base with a pKa of 7.9 at room temperature.[1],[6] Lignocaine with adrenaline is marketed at an acidic pH between 3.3 and 5.5 since its aqueous solubility is higher at this range of pH than at a more physiological pH of around 7.4.[1] Alkalinization of the local anesthetic with sodium bicarbonate reduces the ‘sting’ felt during the injection and hastens the onset time of the anesthetic.[3] It decreases the tissue-injury caused due to the acidic pH of the local anesthetic solution, thus reducing the post-injection soreness. Sodium bicarbonate is clinically applauded because it is easier to work with than carbon dioxide.[6]
The first clinical report of improved onset time of local anesthesia following alkalinization was that of Gros in 1910.[1] Malamed SF[1],[3],[6] has stated that adding sodium bicarbonate to the local anesthetic solution results in a number of clinical advantages. Momota and associates[7] studied the tissue damage by lidocaine and alkalinized lidocaine using a three-dimensional cultured human skin model. The study concluded that alkalinization of lidocaine (pH 7.85, pH 7.9) may have a possibility of decreasing cell viability.
The present study had a similar age distribution in both groups. This is identical to the studies by Gormley[8] and Capogna.[9] The respective mean in our study was 37.45 years and 33.45 years in group A and B. The p value was 0.199 and was found to be non-significant (p> 0.05).
The latency was faster in group B. The mean was 2.0025 minutes in the group B as compared to 2.9505 minutes in group A, which calculated the latency of regular lignocaine with adrenalinesolution. The p value was found to be <0.001 which was highly significant (p <0.01). Our findings are in line with the observations of Al-Sultan and associates[10] who noticed a more rapid onset of action in the buffered group. The findings of our study are also similar to the study by Kashyap and associates[5] who reported a faster onset of anesthesia in the group receiving lignocaine buffered with sodium bicarbonate. Our findings also correlate with Bromage and Gertel[11] who observedthe latency being quicker in carbonated lignocaine Graph 1.
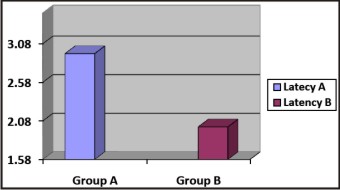 | Graph 1 : Latency (Onset Time)
 |
The findings of the present study go in line with Eppley and Sadove[4] who stated that by increasing the pH (which places it closer to the anesthetic solution pKa) more unionized form is available, that enhances the uptake and makes the onset of anesthesia instantaneous. In our study, the improved latency in group B is probably due to the reason that we used 2% lignocaine with 1: 200,000 adrenaline. Gormley[8] used 1.5% lignocaine with 1: 200,000 adrenaline, while Capogna[9] used 2% plain lignocaine. Our study also supports the findings of Koppal and associates[12] who concluded that alkalinization of lignocaine offers an earlier onset with good intensity and adequate depth of the regional block. Similar increase in onset of alkalinized lignocaine was reported by Mehta and associates[13] in brachial plexus block and Gupta and Kapoor[14] in peribulbar anesthesia.
Sinnot and associates[15] studied the effect of adding sodium bicarbonate to lidocaine with and without epinephrine versus equivalent alkalinization by sodium hydroxide (NaOH) on onset, degree and duration of peripheral nerve block in the rat. They concluded that with 1% commercial lidocaine without epinephrine, sodium bicarbonate decreases the degree and duration of block. However, in solutions with epinephrine, sodium bicarbonate hastens onset, without effecting degree or duration. Our study is in accordance with this finding.
The results of the present study differ from the study by Whitcomb and associates[16] who concluded that buffering 2% lidocaine with 1: 100,000 epinephrine with sodium bicarbonate did not statistically increase anesthetic success, provide faster onset, or result in less pain of injection when compared with unbuffered 2% lidocaine with 1: 100,000 epinephrine for an inferior alveolar nerve block. Our findings also differ from the study by Chow and associates[17], who demonstrated that alkalinization does not hasten the onset of brachial plexus block.
The choice of local anaesthetic will influence the degree to which the pH can be altered without the occurrence of precipitation. Precipitation and pH adjustment study by Peterfreund and associates[18] suggests that lidocaine is particularly suited for alkalinization. This is because it can be alkalinized to a pH close to its dissociation constant without the occurrence of precipitation. DiFazio and associates[19] suggested that improvement in latency is directly related to the degree of change in pH.Gormley and associates[8] had a change of pH from 4.2 in control group to 7.2 in alkalinized group. The study by Capogna and associates[9] had a pH change from 5.85 to 7.12.
In our study the change in pH was from 4.55 to 7.2 in group B, in accordance with Malamed SF.[6] This change in pH after addition of sodium bicarbonate was large enough to achieve the benefits of alkalinization, thereby explaining the earlier onset of anesthesia in Group B. The onset was delayed in group A because it received a solution containing lignocaine with adrenaline without the addition of sodium bicarbonate.
Pain, in the present study, was defined as pain described by the patient on the visual analogue pain scale (VAS) with a 0-10 numeric pain rating scale.[2] Pain was scored before the injection of local anesthesia and after the procedure of alkalinization and anesthesia. The mean VAS before the injection was 8.60 in group A and 8.18 in group B. The VAS before the injection was greater in group A and the p value was 0.197, which was not significant (p> 0.05). The mean VAS after the injection in group A was 2.88 and in group B was 1.93. The VAS after the injection was lesser in group B and the p value was 0.011, which was found to be statistically significant (p< 0.05) Graph 2.
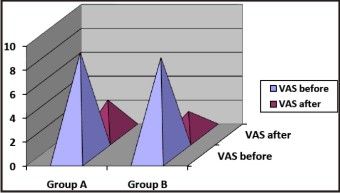 | Graph 2 : Pain On Vas (Before And After)
 |
The final VAS after the injection was obtained by alkalinization of the inflamed pulpal and periapical tissues of the tender tooth. The difference in VAS before and after the injection was highly significant (p <0.01) in both group A and group B with a p value of < 0.001 in each group. The mean change in VAS score in group A was 5.7250 and in group B was 6.2500. The p value was 0.140 (on comparing the changed VAS of the two groups) and was found to be non-significant (p > 0.05). Thus, it can be assumed that both in vivo and ex vivo techniques of alkalinization are equally effective in reducing the pain felt by the patient during the extraction of a tender tooth.
Raymond and associates[20] reported that analgesia upon administration of buffered lidocaine was twice as potent as that of plain lidocaine due to the presence of free CO2 in the alkalinized solution. The present study showed that alkalinization significantly decreased the pain felt during the extraction of infected teeth, a finding that is supported by the study of Al-Sultan and associates[10] who concluded that the pH adjusted solutions enhanced anesthetic efficiency and reduced pain on injection as well as during surgery. Our study upholds the findings of Lem LC[21] who showed that alkalinization of lidocaine decreases the total volume of local anesthetic required and pain of extraction.
The present study used oral antibiotics for the extraction of teeth that remained tender even after alkalinization in both the groups. Out of 40 patients in each group, 10 patients (25%) in group A and 5 patients (12.5%) in group B were prescribed oral antibiotics three times a day and recalled after 2 days for the extraction of the symptomatic tooth. The use of pre-operative antibiotics for the extraction of infected teeth within the two groups individually or in comparison was found to be non-significant (p > 0.05). However, after the tooth extraction, oral antibiotics were prescribed for a period of 5 days along with analgesics (NSAIDs) for the suppression of pain.
The decreased usage of antibiotics before the extraction of tender teeth may suggest a greater quality of anesthesia. It may be seen as a useful alternative to the indiscriminate use of antibiotics prior to the extraction of chronically infected teeth for making the LA solution effective at the site of inflammation. The results of our study support the findings of Gormley[8] and Capogna[9] who showed that the quality was better in pH adjusted group along with an improved latency.
Conclusion
This study concludes that alkalinized local anesthetics produce a more rapid onset of anesthesia than regular local anesthetic solutions and both in vivo and ex vivo techniques of alkalinization are effective in reducing the pain felt by the patient during the extraction of teeth that are tender on percussion. Alkalinization may be used as an adjunct to the pre-operative antibiotic cover prescribed in order to make the local anesthetic effective. Stress-free anesthetic techniques are need of the hour and alkalinization with sodium bicarbonate has a definite role in enhancing patient comfort and clinical performance of the regular local anesthetic solution.
References
1. Malamed SF, Falkel M. Advances in local anesthetics: pH buffering and dissolved CO2. A peer-reviewed CE activity by dentistry today. ADA CERP, June 1, 2009 to May 31, 2012. AGD Pace approval number: 309062.
2. 0–10 Numeric Pain Rating Scale: From McCaffery M, Pasero C. Pain: Clinical Manual, St. Louis, 1999, P. 16. Copyrighted by Mosby, Inc.
3. Malamed SF. Buffering local anesthetics in dentistry. The Pulse volume 44, issue 1, 2011.
4. Eppley BL, SadoveAM. Reduction in injection pain by buffering of local anesthetic solutions. J Oral MaxillolacSurg 47:762.763, 1989.
5. Kashyap VM, Desai R, Reddy PB, Menon S. Effect of alkalinisation of lignocaine for intraoral nerve block on pain during injection, and speed of onset of anaesthesia. BJOMS 49 (2011) e72–e75.
6. Malamed SF. Handbook of Local Anesthesia. 5th ed. St. Louis, MO: Mosby Elsevier; 2004.
7. Momota Y, Imai K, Kishimoto N, Yamabayashi K, Kotani J. Cell viability test for alkalinized lidocaine using the three-dimensional cultured human skin model. AATEX 13(3), 123-126, 2008.
8. Gormley WP, Hill DA, Murray JM, Fee JPH. The effect of alkalinization of lignocaine on axillary brachial plexus anaesthesia. Anaesthesia 1996; 51: 185 – 188.
9. Capogna G, Celleno D, Laudano D, Giunta F. Alkalisation of local anaesthetics. Which block, which local anaesthetic? Regional Anesthesia 1995; 20(5): 369 – 377.
10. Al-Sultan FA, Fathie WK, Hamid RS. A clinical evaluation on the alkalization of local anesthetic solution in periapical surgery. Al-Rafidain Dent J. 2006;6:71-77.
11. Bromage PR, Gertel M. Improved brachial plexus blockade with bupivacaine hydrochloride and carbonated lidocaine. Anesthesiology 1972; 36: 479 – 487.
12. Koppal R, Adarsh ES, Prakashappa DS, Anilkumar G. Comparison of alkalinized and non-alkalinized lignocaine in the brachial plexus block. Journal of Clinical and Diagnostic Research. 2011 December, Vol-5(8): 1610-1613.
13. Mehta R, Verma DD, Gupta V, Gurwara AK. To study the effect of alkalinization of lignocaine hydrochloride on brachial plexus block. Indian J Anaesth. 2003;47:283-286.
14. Gupta RP, Kapoor G. Safety and efficacy of sodium bicarbonate versus hyaluronidase in peribulbaranaesthesia. MJAFI, Vol. 62, No. 2, 2006, pp 116-118.
15. Sinnott CJ, Garfield JM, Thalhammer JG, Strichartz GR. Addition of sodium bicarbonate to lidocaine decreases the duration of peripheral nerve block in the rat. Anesthesiology, V 93, No 4, Oct 2000, pp 1045-52.
16. Whitcomb M, Drum M, Reader A, Nusstein J, Beck M. A prospective, randomized, double-blind study of the anesthetic efficacy of sodium bicarbonate buffered 2% lidocaine with 1: 100,000 epinephrine in inferior alveolar nerve blocks. AnesthProg 57:59-66, 2010.
17. Chow MYH, Alex TH, Kaoy CK, Chan YW. Alkalinization of lidocaine does not hasten the onset of axillary brachial plexus block. Anesthesia and Analgesia 1998; 86: 566–8.
18. Peterfreund RA, Datta S, Ostheimer GW. pH adjustment of local anaesthetic solutions with sodium bicarbonate laboratory evaluation of alkalinization and precipitation. Regional Anesthesia 1989; 14: 265 – 270.
19. DiFazio CA, Carron H, Grosslight KR. Comparison of pH – adjusted lidocaine solutions for epidural anesthesia. Anesthesia and Analgesia 1986; 65: 760 – 764.
20. Raymond S, Wong K, Strichartz G. Mechanisms for potentiation of local anesthetic action by CO2: bicarbonate solutions. Anesthesiology.1989;71(suppl):A711.
21. Lem LC. Efficacy and pain of inferior alveolar nerve block with alkalinized lidocaine. J Oral MaxillofacSurg vol. 49, issue: 8, pp 86, 1991.
|