Introduction
Bacteria have been implicated in the pathogenesis and progression of pulpal and periapical disease. The primary aim of endodontic treatment is to remove as many bacteria as possible from the root canal system and then to create an environment in which any remaining organism cannot survive. This can only be achieved through the use of a combination of aseptic treatment techniques, bio-mechanical preparation of root canal, antimicrobial irrigating solutions and Intracanal medicaments.
Common pathogens which have been implicated in teeth with necrotic pulp and periapical lesions are gram-negative anaerobic bacteria. These gram-negative bacteria are more susceptible to eradication by bio-mechanical procedures than gram-positive, facultative organisms such as Enterococcus faecalis and Staphylococcus spp., which are considered by many researchers to be the most resistant species in the oral cavity and a plausible cause of root canal failure.[1]
Candida albicansis not routinely isolated in primary root canal infections but Waltimo et al, in 1997, reported the presence of Candida albicans in pure cultures in therapy resistant apical periodontitis establishing it as a plausible cause of root canal failure.[2]
These bacteria can exist within the root canal itself or within other related regions such as the dentinal tubules, accessory canals, canal ramifications, apical deltas, fins and transverse anastamoses. Since there is no entirely predictable way to ensure complete elimination of root canal bacteria, an effective antimicrobial agent is required to eradicate or destroy any remaining bacteria.
The need for Intracanal medicament is greater in those cases where bacteria are resistant to routine treatment and where therapy cannot be successfully completed due to presence of pain or continuing exudates.[3] Ideally an Intracanal medicament should also reduce periapical inflammation and consequently reduce pain and induce a cure by formation of hard tissue, control persistent exudation, inhibit osteoclastic activity and prevent or at least delay re-infection of root canal system between sessions. None of the intracanal medicaments used presently, present all these properties which justifies research for newer agents that together with consistent antimicrobial activity are less toxic.
Calcium hydroxide is the most widely used intracanal medicament but its limited activity against Enterococcus faecalis and Candida albicans has put its use as a universal intracanal medicament to question.[3],[4]
Various studies have proved the efficacy of chlorhexidine as an irrigating material, its potential as an Intracanal medicament has also been sought in a gel form in various concentrations.[5],[6],[7]
Chlorhexidine has an added advantage of substantivity, but concern has been expressed regarding the formation of a precipitate containing para-chloroanaline which occludes the dentinal tubules when chlorhexidine combines with sodium hypochlorite which is used as an irrigant. Para-chloroanaline has been shown to be carcinogenic in animals.[8]
Natural products have been used in dental practice for thousands of years and have become even popular today. Propolis is one such product that has attracted increased interest due to its antimicrobial activity against wide range of pathogenic microorganisms. Propolis is a resinous hive product that is collected from various plant sources by bees and the nature of its chemical composition is very complex. The composition of propolis depends upon the vegetation of the area from where it is collected and can vary. Oncag O et al, in an in vitro study found that propolis had good activity against Enterococcus faecalis and suggested its use as an Intracanal medicament.[9] The use of agar diffusion test has been widely used for testing antimicrobial activities of endodontic medicaments.[10],[11] It allows direct comparison of intracanal medicaments against test microorganisms, indicating which medicament has potential to eliminate bacteria from the root canal system.[10]
Thus the purpose of this study was to compare in vitro, the antimicrobial efficacy of 1% & 2% chlorhexidine gel and extract of propolis against pure cultures of Enterococcus faecalis, Candida albicans and Staphylococcus aureus using the agar–well diffusion test.
Materials And Methods
The present investigation was performed against pure lyophilized cultures of Staphylococcus aureus, ATCC6538P, Enterococcus faecalis, ATCC35550 (facultative anaerobes) and Candida albicans ATCC10231 (yeast), as obtained from IMTEC, Ind. The reconstitution of these lyophilized cultures was done on Malt yeast agar for C. albicans and Nutrient agar for E. faecalis and S. aureus. Since the organisms used in this study are not strict anaerobes, they were incubated under aerobic conditions. Mueller-Hinton agar and Mueller-Hinton agar fortified with 2% glucose was used as culture media to test for the antimicrobial efficacy. These agar plates were prepared in sterile glass petri dishes, 90mm in diameter and kept overnight for sterility at 37I0;C.After ensuring sterility, the inoculae of the strains, previously sub-cultured, were prepared with peptone water and the turbidity was compared using the McFarland’s turbidity standard tube No. 0.5. The inoculae were used to make lawn culture of the organisms using sterile cotton swabs on M-H agar, for E. faecalis and S. aureus and on M-H agar with 2% glucose for C. albicans. After making the lawn culture, wells with 6mm diameter and 5mm depth were made in agar plates with sterile Pasteur pipette attached to a vacuum suction. Four wells were made in each agar plate, equidistant from each other and from the rim of the agar plate. The wells were numbered from 1-4 and 1% chlorhexidine gel (ICPA health Ltd, Ind) was added to well no 1, propolis (Apiario Silvestre Green Propolis, Royal Natural Poducts Co., Brasil) was added to well no 2, 2% chlorhexidine gel, (ICPA health Ltd, India) was placed in well no 3 and negative control, propylene glycol (s.d. fine- chem. Limited, Mumbai), which was the vehicle used in all the three test medicaments, was added to well no 4. Ten such agar plates were used for each organism. The plates were incubated overnight at 37I0;C.The specimens were examined after 24 hrs, 72 hrs and 7 days and the zones of inhibition for each chemical used against a particular isolate were recorded with a dial caliper (FORBES, Ltd), calibrated to the least count of 0.02mm. The results were statistically analysed using the one-way ANOVA and post Hoc Turkey HSD.
Results
Statistical analysis of the differences between the mean inhibitory zones for S. aureus, C. albicans and E. faecalis are shown in Table 1.
For S. aureus, at 24 hrs, efficacy of all three test medications was statistically different (p<0.001). 2% chlorhexidine gel showed the maximum inhibitory zone (29.54mm), 1% chlorhexidine gel showed intermediate inhibitory zone (25.09mm) and Propolis showed minimum inhibitory zone (12.82mm). For propylene glycol (control), no inhibition zone was seen. The zones of inhibition remained the same at 24hrs, 72 hrs and 7 days, for all the test medicaments and the control (Table 2).
For E. faecalis, at 24 hrs, efficacy of all the three test medications, was statistically different (p<0.001) with 2% chlorhexidine showing the maximum inhibitory zone(21.84mm) and 1% chlorhexidine showed smaller inhibitory zone(19.70mm). Propolis and the negative control, propylene glycol, showed no zone of inhibition. The zones once obtained remained the same for all test time periods for E. faecalis (Table 3).
For C. albicans at 24 hrs, all the three test medications showed statistically different efficacy (p<0.001). 2% chlorhexidine showed maximum zone of inhibition (27.12mm), 1% chlorhexidine showed smaller zone of inhibition (23.72mm), while propolis and negative control showed no zone of inhibition. However, at 72 hrs, propolis showed a distinct zone of inhibition (11.75mm), which remained the same at 7 days. The zones of inhibition obtained for 1% and 2% chlorhexidine gels remained the same for all test time periods.(Table 4, 5).
E. faecalis was the most resistant organism showing the smallest zones of inhibition, followed by C. albicans, while, S. aureus was the most susceptible with largest zones of inhibition for all test medications at all test time periods. This difference was statistically highly significant (p<0.001) (Graph 1).
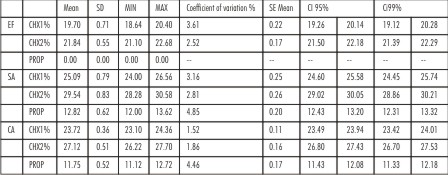 | Table 1 : Mean Zone Diameters with standard deviation for Enterococcus faecalis (EF), Staphylococcus aureus (SA) and Candida albicans (CA) after 7 days, for 1% Chlorhexidine gel (CHX1), 2% Chlorhexidine gel (CHX2) and Propolis (PROP) Also showing, Minimum
 |
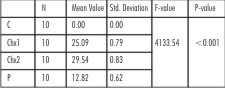 | Table 2 : One-way ANOVA for the comparison of mean zone of inhibition (in mm), for Propylene glycol, Control (C), 1% Chlorhexidine gel (CHX1), 2% Chlorhexidine gel (CHX2) and Propolis (P) for Staphylococcus aureus at 24 hrs, 72 hrs and 7 days.
 |
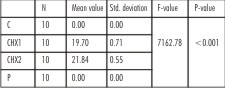 | Table 3 : One-way ANOVA for the comparison of mean zone of inhibition (in mm) for Propylene glycol, Control (C), 1% Chlorhexidine gel (CHX1), 2% Chlorhexidine gel (CHX2) and Propolis (P), for Enterococcus faecalis at 24 hours, 72 hours and 7 days
 |
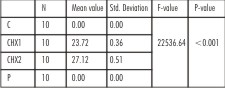 | Table 4 : One-way ANOVA for the comparison of mean zone of inhibition (in mm), for Propylene glycol, Control (C), 1% Chlorhexidine gel (CHX1), 2% Chlorhexidine gel (CHX2) and Propolis (P) for Candida albicans at 24 hours
 |
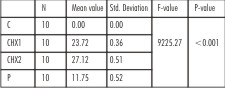 | Table 5 : One-way ANOVA for the comparison of mean zone of inhibition (in mm), for Propylene glycol, Control (C), 1% Chlorhexidine gel (CHX1), 2% Chlorhexidine gel (CHX2) and Propolis (P) for Candida albicans at 72 hrs and 7 days.
 |
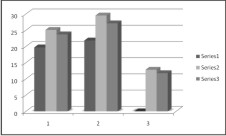 | Graph 1 : 1- Comparative zones of inhibition formed by Chlorhexidine 1%, 2- Comparative zones of inhibition formed by Chlorhexidine 2%, 3- Comparative zones of inhibition formed by Propolis, Series 1- Enterococcus faecalis, Series 2- Staphylococcus aureus
 |
Discussion
The present study investigated the antimicrobial efficacy of 1% and 2% chlorhexidine gels and propolis against E. faecalis, S. aureus and C. albicans. In a study by Lin et al[12] (2003) it was stated that chlorhexidine should remain within the root canal for 7 days in order to reach a distance of 500 microns within the tubules to achieve adequate disinfection, as E. faecalis can reach the tubules up to a depth of 300-400 microns after a 3-week incubation period[13] and a depth of 500-700 microns after 60 days of incubation.[14] It is also not always possible to complete the root canal treatment in a single sitting, so an inter- appointment dressing is needed to keep the canal free of the bacteria within appointments.
Therefore, the short term and long term effect of the medicaments was evaluated for 24 hrs, 72 hrs and 7 days.
Mueller-Hinton agar was used for testing the efficacy of the medicaments as it has a neutral pH (7.2-7.4), and does not affect the efficacy of the medicament due to a difference in pH of the medium. 2% glucose was added to Mueller-Hinton agar for inoculation of C. albicans, since C. albicans is a slow growing organism and an enriched media is required to obtain its growth in subculture. The inoculum was standardized to 0.5 McFarland scale using a barium sulphate standard, so that each time a fixed number of microorganisms were seeded onto the agar plates. There is no universal agreement on the size of the wells made in agar, but the usual antibiotic discs used in antibiotic sensitivity testing have a fixed diameter of 6mm, therefore, the well size was standardized to 6mm diameter and 5mm depth of the agar medium and fixed amount of medicament, i.e. 50 micro-litres was added to each well made in agar, in order to reduce the chances of error owing to difference in the amount of medication or size of sample.[15]
Chlorhexidine was used as it has multiple benefits, one of the main benefit being its substantivity which is conferred to the root dentin for a period of 12 weeks according to Rosenthal et al (2004).[16] Unlike the conventional medicaments, the positively charged molecules of chlorhexidine can adsorb onto dentin and prevent microbial colonization due to its substantive property. It can also be used in cases where the root formation is incomplete due to its marked bio-compatibility.
Chlorhexidine is a cationic bisguanide that seems to act by adsorbing onto the cell wall of the microorganisms and causing leakage of intracellular components. The miminum inhibitory concentration (MIC) of chlorhexidine has been reported to be up to 256 microgram/ml for aerobic and facultative anaerobic microorganisms.[17]
At lower concentration, small molecule weight substances leak out, resulting in a bacteriostatic effect. At higher concentration, it has a bactericidal effect due to the precipitation and/or coagulation of the cytoplasm, probably caused by protein cross-linking. Thus, the effect of chlorhexidine is proportional to its concentration, as greater numbers of chlorhexidine molecules are available by using higher concentrations.[18]
In the present study, chlorhexidine concentration of 1% and 2% is much higher than the minimum inhibitory concentration for facultative anaerobic and aerobic microorganisms. So, the effect of 1% chlorhexidine gel and 2% chlorhexidine gel seen in the present study is essentially bactericidal in nature with 2% chlorhexidine gel showing better efficacy. The concentration of chlorhexidine upto 2% is considered to be biologically safe.[1]
Propolis showed inhibition against S. aureus at all test time periods.Takaisi-Kikuni and Schilcher observed the mode of action of propolis on Streptococcus agalactiae, by electron microscopic and micro-calorimetric modes. They observed that propolis inhibited bacterial growth by preventing cell division. In addition, propolis disorganized the cytoplasm, cytoplasmic membrane and the cell wall, caused a partial bacteriolysis and inhibited protein synthesis.[19]
There was no activity of propolis seen at 24 hrs for Candida albicans. Faint growth of the yeast was seen at 24 hrs within the halo formed by diffusion of propolis. While at 72 hrs, thick Candidal growth was observed on the agar plates except a well demarcated zone around propolis in which faint growth that was seen at 24 hrs persisted. This zone remained unchanged at 7 days, with no growth of the yeast cells within the halo. A possible explanation for this may be that propolis exerted a fungistatic activity against Candida albicans and the effect of the medicament did not appear for several generation times, so faint growth was seen at 24 hrs which was soon halted by the action of propolis, as checked at 72 hrs and till the 7 day time period.[20]
The possible mechanism of the antifungal action was studied by Mello et al. It was suggested that the effect of propolis on fungi was attributed to its interaction with cellular sulphydryl compounds, thus having a deleterious effect on the integrity of the cell wall.[21]
Propolis as a medicament showed no zone of inhibition for Enterococcus faecalis at all time periods. A study by Kosalec et al (2005), in which the flavonoid content and the antimicrobial activity was evaluated for ten commercially available ethanolic solutions of propolis, on six bacteria and one yeast showed that, the largest zones of inhibition were obtained for Staphylococcus aureus and the smallest for Enterococcus faecalis with some extracts showing no activity on Enterococcus faecalis. It was concluded that this was due to a low concentration of bactericidal flavonoids in the product.[22]
In the present study as well, the lower content of flavonoids in the propolis sample used, or their reduced solubility in propylene glycol used as a solvent, or diffusibility through the agar medium may have been responsible for the lack of effectivity against Enterococcus faecalis.
In-vitro studies have their limitations. Endodontic infections are primarily polymicrobial. The medicament that is effective against a single microbe in vitro, may not necessarily be effective against the same microbe in vivo since the root canal system has multiple microorganisms in a well organized bio-film. Also, the buffering capacity of dentin, the presence of exudates, all has a bearing on the effectivity of the medication.
Agar diffusion test is a simple and inexpensive test for the comparison of the drugs having a similar diffusion gradient. The present study wherein propylene glycol was the vehicle for all test medicaments, justifies the direct comparison of the efficacy of the medicaments by use of the agar diffusion test.
But the results of the agar diffusion method, depend upon the molecular size, solubility and diffusion of the materials through the aqueous agar medium, the sensitivity of the drug, the bacterial source (wild strain or collection sample), the number of bacteria inoculated, the pH of the substrates in plates, agar viscosity, storage conditions of agar plates, incubation time and metabolic activity of the microorganisms.[11]
Thus the results of the agar-diffusion test cannot be directly extrapolated to the clinical conditions.In the agar diffusion test, the size of the microbial inhibition zone depends upon the solubility and diffusibility of the test substance and, therefore, may not express the medicament’s full effective potential. The direct exposure test is correlated to substance effectiveness and has direct contact with the microorganisms, it seems to be independent of the other variables and appears to be a practical laboratory test as well. Clinical trails using propolis as an intracanal medicament on failed endodontic cases or persistent non-healing lesions would give a better understanding on the effectiveness of propolis.
Also, before accepting or abandoning propolis as an intracanal medicament, it becomes imperative to understand that the flavonoid composition of propolis is both quantitatively and qualitatively different, depending on the region from where it is collected. Thus, the activity of propolis may also vary. Therefore, it would be important to set guidelines of standardization and quality control of crude form of propolis, which is used to manufacture dental products.
Conclusion
The present study suggests that 2% chlorhexidine gel as intracanal medicament has a significant antimicrobial effect against E. faecalis, C. albicans and S. aureus. It is more effective than 1% chlorhexidine gel and Propolis for 7 days.
Refrences
1. Gomes BPFA, Lilley JD, Drucker DB. Variations in the susceptibilities of components of the endodontic microflora to biomechanical procedures. Int Endod J 1996; 29:235-41.
2. Waltimo TMT, Siren EK, Torkko HLK, Olsen I, Haapasalo MPP. Fungi in therapy resistant apical periodontitis. Int Endod J 1997; 30:96-101.
3. Gomes BPFA, Souza SFC, Ferraz CCR, Teixeira FB, Zaia AA, Valdrighi L, Souza-Filho FJ. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J 2003; 36: 267-75.
4. Basrani B, Tjaderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, Friedman S. Efficacy of chlorhexidine and calcium hydroxide containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96(5):618-24.
5. Almyroudi A, Mackenzie D, McHugh S, Saunders WP. The effectiveness of various disinfectants used as intracanal medications: An in vitro study. J Endod 2002; 28 (3):163-7.
6. Gomes BPFA, Vianna ME, Zaia AA, Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as an intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 102(4):544-50.
7. Basrani B, Ghanem A, Tjaderhane L. Physical and chemical properties of chlorhexidine and calcium hydroxide containing medications. J Endod 2004; 30(6):413-7.
8. Bui TB, Baumgartner JC, Mitchell JC. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod 2008; 34(2):181-5.
9. Oncag O, Cogulu D, Uzel A, Sorkun K. Efficacy of propolis as an intracanal medicament against Enterococcus faecalis. Gen Dent 2006; 54(5):319-22.
10. Siqueira Jr. JF, Uzeda M. Intracanal Medicaments: Evaluation of the antibacterial effects of chlorhexidine, metronidazole and calcium hydroxide associated with three vehicles. J Endod 1997; 23(3):167-9.
11. Gomes BPFA, Ferraz CCR, Vianna ME, Rosalen PL, Zaia AA, Teixeira FB, Souza-Filho FJ. In vitro Antimicrobial activity of calcium hydroxide pastes and their vehicles against selected microorganisms. Braz Dent J 2002; 13(3):155-61.
12. Lin S, Zukerman O, Weiss El, Mazor Y, Fuss Z. Antibacterial efficacy of a new chlorhexidine slow release device to disinfect dentinal tubules. J Endod 2003; 29: 416-8.
13. Saleh IM, Ryuter IE, Haapasalo M, Orstavik D. Survival of Enterococcus faecalis in infected dentinal tubules after root canal filling with different root canal sealers in vitro. Int Endod J 2004; 37: 193-8.
14. Gomes NV, Gurgel-Filho ED, Gomes BPFA, Ferraz CCR, Zaia AA, Souza-Filho FJ. Recovery of Enterococcus faecalis after single or multiple visit root canal treatments carried out in infected teeth ex vivo. Int Endod J 2005; 38: 697-704.
15. World Health Organization. Chapter17- In vitro susceptibility of bacteria to antimicrobial agents (document on internet). 2009 (updated 2009 Nov 2; cited 2009, Nov19). Available from: http://www.whoindia.org/CDS/LabNet/Std-Guidelines/IDSP%20Lab%20Manual/NICD-1.PDF
16. Rosenthal S, Spanberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004; 98(4):488-92.
17. Emilson CG. Susceptibility of various microorganisms to chlorhexidine. Scand J Dent Res 1997; 85:255-65.
18. Gomes BPFA, Ferraz CCR, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J 2001; 34:424-8.
19. Takaisi-Kikuni NB, Schilcher H. Electron microscopic and micro-calorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med 1994; 60:222-7.
20. Arvidson S, Dornbusch K, Ericsson H. Interpretation of the agar diffusion method for bacterial susceptibility testing. J Antimicrob Chemother 1981; 7:5-14
21. Mello AM, Gomes RT, Lara SR, Silva LG, Alves JB, Cortes ME, et al. The effect of Brazilian propolis on the germ tube formation and cell wall of Candida albicans. Pharmacologyonline 2006; 3:352-8.
22. Kosalec I, Pepeljnjak S, Bakmaz M, Knezevic SV. Flavonoid analysis and antimicrobial activity of commercially available propolis products. Acta Pharm 2005; 55:423-430.
|