Introduction
Stem cell is defined as a cell that has the ability to continuously divide and produce progeny cells that differentiate (develop) into various other types of cells or types of tissues. Most of the 300 trillion cells of the body have completely specialized functions. Blood, lung, brain, skin or liver cells are all wonderfully specialized for what they do. By and large, they cannot do anything other than what they were designed for. On the other hand, stem cells exist mainly to maintain and repair cells in the areas where they are found. Stem cells are found in the blood, bone marrow, muscle, skin, and organs like the brain and liver.[1]
History
The term stem cell was proposed for scientific use by Russian histologist Alexander Maksimov in 1908. Research on stem cells grew out by Canadian scientists in the 1960s.The history of stem cell research had a benign, embryonic beginning in the mid 1800's with the discovery that some cells could generate other cells. In the early 1900's real stem cells were discovered and it was found that some cells generate blood cells. The history of stem cell research includes work with both animal and human stem cells. A prominent application of stem cell research has been bone marrow transplants using adult stem cells. In the early 1900's physicians administered bone marrow by mouth to patients with anemia and leukemia. Although such therapy was unsuccessful, laboratory experiments eventually demonstrated that mice with defective marrow could be restored to health with infusions into the blood stream of marrow taken from other mice. This caused physicians to speculate whether it was feasible to transplant bone marrow from one human to another (allogenic transplant). Among early attempts to do this were several transplants carried out in France following a radiation accident in the late 1950's. Performing marrow transplants in humans was not attempted on a larger scale until a French medical researcher made a critical medical discovery about the human immune system. It was not until the 1960's that physicians knew enough about HLA compatibility to perform transplants between siblings who were not identical twins. The 1990's saw rapid expansion and success of the bone marrow program with more than 16,000 transplants to date for the treatment of immunodeficiencies and leukemia. In 1998, James Thompson (University of Wisconsin-Madison) isolated cells from the inner cell mass of early embryos, and developed the first embryonic stem cell lines. In the same year, John Gearhart(Johns Hopkins University) derived germ cells from cells in fetal gonadal tissue (primordial germ cells). Pluripotent stem cell "lines" were developed from both sources. Ongoing researches are now focused on transplanting stem cells of non-self origin.[1]
How do stem cells differ from other cells in the body?
All stem cells, regardless of their source, have three general properties, which make them different from other cells in the body:
(1) They are capable of dividing and renewing themselves for long periods:
Unlike muscle cells, blood cells, or nerve cells – which normally do not replicate themselves – stem cells may replicate many times, called proliferation. A starting population of stem cells that proliferates for many months in the laboratory can yield millions of cells. If the resulting cells continue to be unspecialized, like the parent stem cells, the cells are said to be capable of long-term self-renewal.
(2) They are unspecialized:
One of the fundamental properties of a stem cell is that it does not have any tissue-specific structures that allow it to perform specialized functions. However, unspecialized stem cells can give rise to specialized cells, including heart muscle cells, blood cells, or nerve cells.
(3) They can give rise to specialized cell types:
When unspecialized stem cells give rise to specialized cells, the process is called differentiation. Scientists are just beginning to understand the signals inside and outside cells that trigger stem cell differentiation
Stem cells are of two types-
- embryonic/fetal and
- adult/postnatal.
Embryonic stem cells are derived from embryos generated by in vitro fertilization. Whereas postnatal stem cells are derived from umbilical cord blood, umbilical cord, bone marrow, peripheral blood, brain, eyes, liver, muscles, skin almost all parts of the body tissues including the pulp tissues of teeth. The reason why it is important to distinguish between embryonic and postnatal stem cells is because these cells have a different potential for developing into various specialized cells i.e. plasticity. The plasticity of the stem cell defines its ability to produce cells of different tissues. The reason why it is important to distinguish between embryonic and postnatal stem cells is because these cells have a different potential for developing into various specialized cells, i.e. plasticity.[2],[3],[4]
Current Scope of Applications of Stem Cells in Dentistry:[1],[5],[6],[8]
1. In continued root formation
2. In pulp healing and regeneration
3. In replantation and transplantation
4. Pulp/dentin tissue engineering and regeneration
5. Bioroot engineering and reconstruction of the periodontium.
Potential Role of Stem Cells in Continued Root Formation
Using minipigs as a model, a pilot experiment was conducted Although the finding suggests that root apical papilla is likely to play a pivotal role in root formation, further research is needed to verify the role of stem cells from apical papilla in continued root formation.
Potential Role of Stem Cells in Pulp Healing and Regeneration
Recent researches challenge the traditional approach in managing immature teeth by applying apexification treatment, where there is little to no expectation of continued root development. Instead, it is possible that alternative biologically-based treatments may promote apexogenesis / maturogenesis. A common aspect of many of these reported cases is the preoperative presentation of apical periodontitis with sinus tract formation, a condition normally associated with total pulpal necrosis and infection that requires apexification.
Although Iwaya et al and Banchs and Trope applied the term 'revascularization' to describe this phenomenon, what actually occurred was physiological tissue formation and regeneration initiated by the stem cells .
Potential Role of Stem Cells in Replantation and Transplantation
Andreasen et al and Kling et al showed excellent radiographic images of the ingrowth of bone and periodontal ligament (PDL) (next to the inner dentinal wall) into the canal space with arrested root formation after the replantation of avulsed maxillary incisors, suggesting a complete loss of the viability of pulp, apical papilla, and/of HERS. Skoglund et al observed revascularization of' the pulp of replanted and autotransplanted teeth with incomplete root development in dogs. Ingrowth of new vessels occurs during the first few postoperative days. After 10 days, new vessels are formed in the apical half of the pulp and, after 30 days, in the whole pulp.
Stem Cells for Pulp/Dentin Tissue Engineering and Regeneration
Dental pulp tissue engineering was first tested by Mooney's groups. Bohl et al reported that culturing pulp cells grown on poly glycolic acid (PGA) in vitro resulted in high cell density tissue similar to the native pulp. Buurma et al found that pulp cells seeded in PGA and implanted into the subcutaneous space of immunocompromised mice produced extracellular matrix. New blood vessels also penetrated the cells/PGA implants in vivo 3 weeks after the implantation. Since, the isolation and characterization of DPSCs and SHED using these stem cells for dentin/pulp tissue regeneration has drawn great interest. These findings provide new light on the possibility of generating pulp and dentin in pulpless canals.
Stem Cells for Bioroot Engineering [10]
Dental implants have recently gained momentum as a preferred option for replacing missing teeth instead of bridges or removable dentures. However, although dental implants have had great improvements over the past decades, the fundamental pitfall is the lack of a natural structural relationship with the alveolar bone (i.e. the absence of PDL). In fact, it requires a direct integration with bone onto its surface as the prerequisite for success, an unnatural relation with bone as compared with a natural tooth. The lack of natural contours and its structural interaction with the alveolar bone make dental implants a temporary option until a better alternative is available. This alternative may be tooth regeneration. Using animal study models, cells isolated from tooth buds can be seeded onto scaffolds and form ectopic teeth in vivo. Nakao et al recently engineered teeth ectopically followed by transplantation into an orthotopic site in the mouse jaw. Tooth regeneration at orthotopic sites using larger animals such as dogs and swine has also been tested. The study in dogs failed to show root formation whereas the swine model was able to show root formation with a 33.3% success.
Overall Uses Of Stem cells: (Fig.1):
Stem cells in mammalian development: (Fig 2)
 | Overall Uses Of Stem cells: (Fig.1)
 |
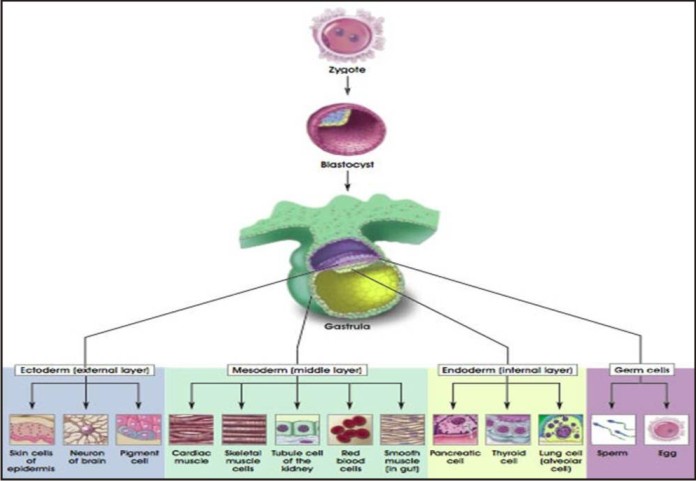 | Stem cells in mammalian development: (Fig 2)
 |
The zygote is the ultimate stem cell. It is totipotent with the ability to produce all the cell types of the species including the trophoblast and the embryonic membranes. Development begins when the zygote undergoes several successive cell divisions, each resulting in a doubling of the cell number and a reduction in the cell size. At the 32 to 64-cell stage each cell is called a blastomere
The blastomeres stick together to form a tight ball of cells called a morula. Each of these cells retains totipotential. The next stage is the blastocyst which consists of a hollow ball of cells; trophoblast cells along the periphery develop into the embryonic membranes and placenta while the inner cell mass develops into the fetus. Beyond the blastocyst stage, development is characterized by cell migration in addition to cell division. The gastrula is composed of three germ layers : the ectoderm, mesoderm and endoderm. The ectoderm is the outer layer ,It gives rise to future nervous system and the epidermis (skin and associated organs such as hair and nails). The mesoderm is middle layer. It gives rise to the connective tissue, muscles, bones and blood. The endoderm is the inner layer. It forms the gastrointestinal tract of the future mammal. Early in embryogenesis, some cells migrate to the primitive gonad or genital ridge. These are the precursors to the gonad of the organism and are called germinal cells. These cells are not derived from any of the three germ layers but appear to be set aside earlier.
The role of adult stem cells in tissue repair
During development, stem cells divide and produce more specialized cells. Stem cells are also present in the adult in far lesser numbers. The role of adult stem cells (also called somatic stem cells) is believed to be replacement of damaged and injured tissue. Observed in continually replenished cells such as blood cells and skin cells, stem cells have recently been found in other tissue, such as neural tissue. Organ regeneration has long been believed to be through organ-specific and tissue-specific stem cells. Hematopoietic stem cells were believed to replenish blood cells, stem cells of the gut to replace cells of the gut and so on. Recently, using cell lineage tracking, stem cells from one organ have been discovered that divide to form cells of another organ. Hematopoietic stem cells can give rise to liver, brain and kidney cells. This plasticity of adult stem cells has been observed not only under experimental conditions, but also in people who have received bone marrow transplants.
Role of stem cells in cancer[7],[9]
Ontogeny (development of an organism) and oncology (cancer development) share many common features. From the 1870s the connection between development and cancer has been reported for various types of cancers. Existence of "cancer stem cells" with aberrant cell division has also been reported more recently. The connection between cancer and development is clearly evident in teratocarcinomas. As early as 1862, Virchow discovered that the germ cell tumor teratocarcinoma is made up of embryonic cells. In 1970, Stevens derived embryonal carcinoma cells from teratocarcinomas. A teratocarcinoma is a spontaneous tumor of germ cells that resembles development gone awry. This tumor may contain several types of epithelia: areas of bone, cartilage, muscle, fat, hair, yolk sac, and placenta These specialized tissues are often adjacent to an area of rapidly dividing unspecialized cells. The teratocarcinomas are able to differentiate into normal mature cells when transplanted into another animal. This alternation between developmental and tumor cells status demonstrates how closely development and cancer are related. McCulloch explored the connection between normal development of blood cells and leukemia. According to him, normal hematopoietic development requires the interaction of stem cell factor with its receptor, c-kit. A hierarchy of stem and progenitor cells differentiates and produces different sub lineages of cells resulting from response to varied growth factors.
Malignancies of the hematopoietic system originate from two sources:
those with an increased growth in an early stem cell produce acute leukemia, while those that arise from a decreased response to death or differentiation in a stem cell produce chronic leukemia.
Stem cells used in research and clinical applications[9],[11]
Types and characteristics of stem cells for culture:
Embryonic stem (ES) cells are obtained from the inner cell mass and cultured. ES cells are the best characterized of all the cultured stem cells.
Properties of ES cells:
(i) ES cells are pleuripotent, i.e. they have the ability to differentiate into cells derived from all three germ layers, but not the embryonic membranes.
(ii) ES cells are immortal i.e. cells proliferate in culture and have been maintained in culture for several hundred doublings.
The advantage of maintaining stem cells in culture is that they are a source of a large number of cells in the undifferentiated state.
So far other adult stem cells have not been maintained indefinitely.
(iii)ES cells maintain a normal karyotype.(there are no major structural changes in the chromosomes)
(iv) ES cells display Oct-4 protein and other unique markers on the cell surface.
ES cells can be induced to differentiate in vitro by culturing in suspension to form three-dimensional cell aggregates called embryoid bodies (Ebs). The cells spontaneously differentiate into various cell types. e.g. neurons, cardiomyocytes, and pancreatic beta cells. The addition of growth factors to the culture directs differentiation to specific cell types. However, it is still challenging to isolate pure differentiated cell types. Following injection of ES cells into immunodeficient mice, teratomas develop with derivatives of all three germ layers. This is a major disadvantage of using ES cells for cell therapy since any contaminating undifferentiated cells could give rise to cancer.
Conclusion:
Adult stem cells offer hope for cell therapy to treat diseases in the future because ethical issues do not impede their use. In addition, if the patient's own cells are used, immunological compatibility is not an issue. However, ES cells have been found to be superior for both differentiation potential and ability to divide in culture. [12]
References:
1. Krishna Vyas,Madathanapalli Satish,Vaishali Shende,Rahul Srivastav,Stem Cells-The Future Of Dentistry: A Review, JIAOMR, July-Sep 2011;23(3):S370-372
2. Dr. Shantanu Khatri,Dr.Madhavi Bhardhwaj,Stem Cells:Where the future lies,The Orthodontic Cyber Journal,Jan-2010
3. Siminovitch L,Mcculloch E A,Till J E.The distribution of colony forming cells among spleen colonies.J Cell Physiol.1963 Dec;62:327-36.
4. Becker AJ,Mcculloch E A, Till J E.Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells.Nature.1963 Feb 2;197:452-4
5. Ramesh Reddy,Mahesh Babu,V.Sasidhar Reddy,P.Gautham.Stem Cells in Dentistry-A Revolution in Regeneration.Medico-legal update.Jan-June,2012,Vol.12,No.1
6. Peter E. Murra, Franklin Garcia-Godoy,Kenneth M. Hargreaves. Regenerative Endodontics: A Review of Current Status and a Call for Action, JOE—Volume 33, Number 4, April 2007
7. Tatiana Jazedje,Mariane Secco et al.Stem cells from umbilical cord blood do have myogenic potential,with and without differentiation induction in vitro.J Transl Med.2009;7:6.
8. Amit Gandhi,Taru Gandhi,Natasha Madan. Dental pulp stem cells in endodontic research: a promising tool for tooth tissue engineering, RSBO. 2011 Jul-Sep;8(3):335-40 – 337
9. Matzilevich D. Mesenchymal Stem Cells-Sources and clinical Applications.Transfusion Medicine and Hemotherapy 2008:35;4
10. G. Bluteau, H-U. Luder, C. De Bari, T. A. Mitsiadis. Stem Cells For Tooth Engineering,European cells and Materials Vol. 16,2008(1-9).
11. Brett Peterson,Jeffery Zhang et al.Healing of Critically Sized Femoral Defects,Using Genetically Modified Mesenchymal Stem Cells from Human Adipose Tissue.Tissue engineering.2005;11;120-129.
12. Hans Klingermann.Discarded stem cells with a future?Expert Opinion on Biological Therapy,2006;6:1251-1254.
|