Introduction:
Aggressive periodontitis comprises a rare group of disease characterized by rapid clinical attachment and alveolar bone loss in young systemically healthy individuals[1], with the amount of plaque and calculus on the affected teeth inconsistent with the severity of periodontal destruction, as opposed to chronic periodontitis.[2] This disease has undergonea series of terminology changes over the years to be finally named as “aggressive periodontitis”.[1] Various theories about pathogenesis of aggressive periodontitis have evolved from a purely plaque associated disease to the more recent hypotheses, emphasising on the host’s response to bacteria.[3],[4]
The host-derived pro-inflammatory mediators likecytokines- IL-1β & TNF-α released after bacterial infection are the potent inducers of bone resorption & inhibitors of bone formation.[5] Anti-inflammatory cytokines, like IL-4 and IL-10 contributes to negative immune regulation by down regulating the production of other proinflammatory cytokines.[6] The aim of this study was to investigate the shift in levels of selected pro and anti-inflammatory cytokines (IL-1β, IL-2, IL-6, TNF-α, IL-4, IL-10 and GM-CSF) in GCF and peripheral blood in diagnosed cases of generalized aggressive periodontitis at baseline and 3 & 6 months after periodontal surgical treatment compared to healthy volunteers.
Material And Methods:
A 6 month case controlled clinical and experimental study for the evaluation of the level of cytokines in GCF and peripheral blood of GAgP patients was conducted in the Department of Periodontics, Babu Banarasi Das College of Dental Sciences, Lucknow, Uttar Pradesh in collaboration with, Indian Institute of Toxicology Research (IITR), Lucknow.
Study Design
The patients visiting the Out Patient Department of the Dental College were screened and a total of 24 volunteers irrespective of cast, creed and sex between the age range of 20-50 years were selected. They were divided into two groups, Group A consisting of 12 subjects with GAgP, demonstrating a minimum of 20 natural teeth present; with Probing Pocket Depth and Clinical Attachment Level > 5 mm at a minimum of one site in more than 10 teethand Group B with 12 subjects who were periodontally and systemically healthy, having a minimum of 24 natural teeth with PPD and CAL ≤ 3 mm and less than 20% of the sites with bleeding on probing.
Subjects with significant systemic disease, those who had received antibiotic therapy within the last 3 month and periodontal treatment within last 6 month, pregnant or lactating women, smokers, tobacco chewers and alcoholics were excluded.
The study has been approved by the Institutional Ethics Committee. Written informed consent was obtained from each subject prior to entering the study.
Clinical Assessments
Periodontal examinations were performed by a single examiner, the following parameters were recorded to the nearest millimeter at four sites in each teeth (mesiobuccal, midbuccal, distobuccal, midlingual / midpalatal) using a manual periodontal probe (University of North Carolina–Hu-Friedy, USA): Plaque Index (PI): (Silness and Loe, 1964); Gingival Index (GI): (Loe and Silness, 1963); Probing pocket depth (PPD) and Clinical Attachment Level (CAL).
Gingival Crevicular Fluid Sampling
GCF was collected from 4 sites with deepest periodontal pockets from each volunteer. After removing the supragingival biofilm with sterile cotton pellets, absorbent paper points number 40 were inserted into the orifice of the periodontal pocket until a slight resistance was felt and then left for 30 seconds. Points contaminated with blood were discarded. They were placed in Eppendorftube containing 250 μl of RPMI-1640 transport media and immediately transported to IITR, Lucknow in a minicooler, maintaining a temperature of -200C, where they were stored at a temperature of – 800C for the cytokine analysis. (Fig - 1, 2, 3).
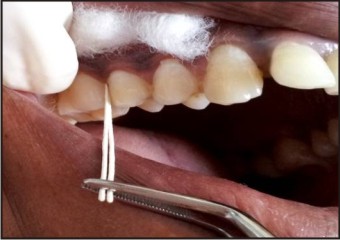 | Fig 1 : GCF collection
 |
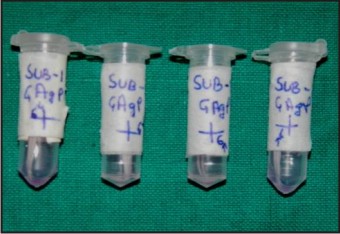 | Fig 2 : Paper Points after GCF collection (Kept in transport media)
 |
 | Fig 3 : Mini cooler (for transportation of GCF samples)
 |
Collection of Peripheral Blood
5 ml intravenous blood was collected from the anticubital vein of each volunteer and was divided into 2 parts: 2.5 ml in BD Vacutainer with Sodium Heparin and remaining 2.5 ml in microcentrifuge tube for separation of plasma. Following collection, the samples were kept on ice, maintaining the temperature 0-40 C and immediately transported to IITR, stored at - 4°C and used for subsequent assays. (Fig - 4, 5, 6).
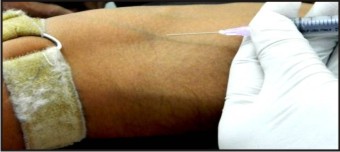 | Fig 4 : Peripheral Blood collection
 |
 | Fig 5 : Blood collected in vacutainer with sodium heparin
 |
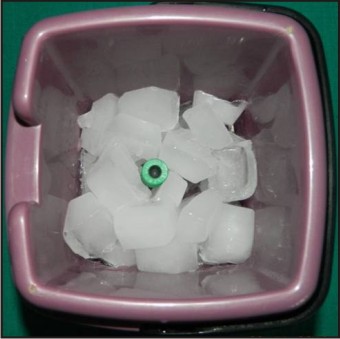 | Fig 6 : Blood sample kept in ice for transportation
 |
Periodontal Treatment Procedure
All the GAgP subjects underwent phase-I therapy after collection of samples and chlorhexidine mouth rinse 0.2% twice daily was advised. After a re-evaluation period of 4 weeks periodontal surgical procedure was carried out. Antimicrobial therapy – Doxycycline (100 mg) 2 tabs stat followed by 1 tab once daily for 10 days and an anti-inflammatory drug was prescribed twice daily for 3 days along with Chlorhexidine mouthwash 0.2% twice daily for 2 weeks.
After 3 and 6 months post treatment, all the parameters were repeated and again subjected to analysis.
Quantification of cytokines in GCF and Peripheral blood
Separate ELISA kits for each cytokine by eBioscience (San Diego, USA) were used for quantification of the levels of plasma and GCF cytokines as per the protocol provided by the manufacturer.
Statistical Methods
Data were summarized as Mean±SD. The variables between Group A and Group B were compared by Mann-Whitney U test. Wilcoxon rank sum test was used to compare the variables from baseline to 3 & 6 month and 3 to 6 month in Group A. The p-value less than 0.05 were considered as significant.
Results
Clinical Parameters
Table 1 presents the clinical parameters for the two groups at baseline and at 3 and 6 month.PPD and CAL were significantlyhigher among the patients of Group A than Group B at baseline, with a statisticaly significant reduction from baseline to 3and 6 monthin Group A.An insignificant difference was seen in PI and GI between both the groups at baseline. However there was astatistically significant reduction in both PI and GIseen in Group A when compared to Group B at 6 month.
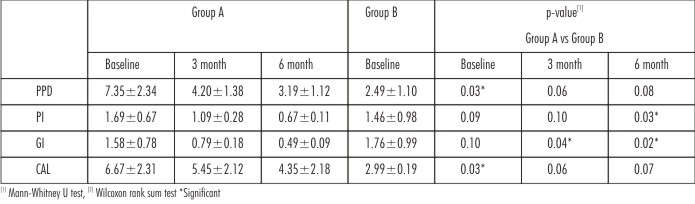 | Table 1 : Comparison Of Clinial Parameters
 |
Cytokine levels in GCF
The levelsof cytokines in both the groups at baseline, 3 and 6 months are summarized in Table 2. All the cytokines were seen to be elevated in GAgP subjects at baseline when compared to controls. There was a significant differencein the level of IL-1β between both the groupsat 3 month, whereasnone of the other GCF cytokines were differentat 3 and 6 month after treatment.
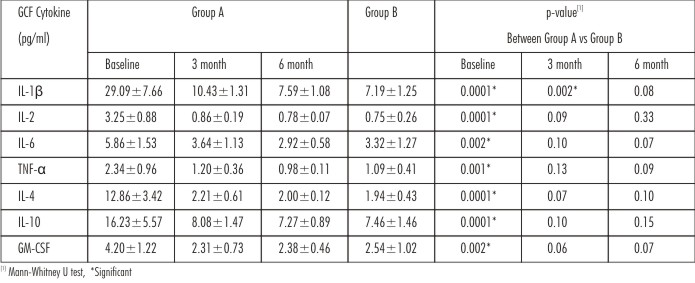 | Table 2 : Comparison Of Gcf Cytokine (Pg/Ml) Level Between Group A And Group B At Baseline, 3 Months And 6 Months Post Treatment
 |
Cytokine levels in Peripheral blood
The levelsof cytokines in both the groups at baseline, 3 and 6 months are summarized in Table 3. There was a significant difference in the levels of IL-1β, IL-2, IL-6 and IL-10 between the groups at baseline with a significant difference in IL-1β level between both the groups at 3 and 6 month. None of the other blood cytokines were differentat 3 and 6 months between the groups. There was no significant difference for TNF-α, IL-4 and GM-CSF between the groups at any point of time of the study.
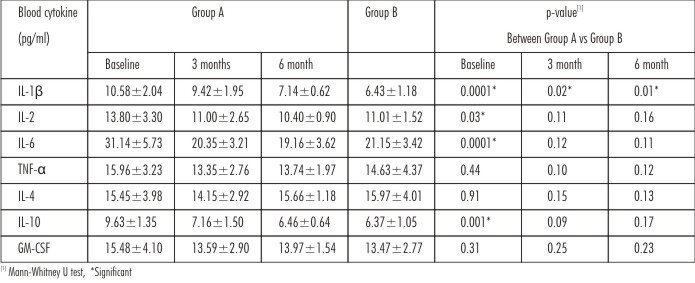 | Table 3 : Comparison Of Peripheral Blood Cytokine (Pg/Ml) Level Between Group A And Group B At Baseline, 3 Months And 6 Months Post Treatment.
 |
Discussion
Specific bacterial etiology with the predominance of Aggregatibacter actinomycetemcomitansandPorphyromonas gingivalis[7],[8]has been associated with GAgP.Studies documented in literature that have investigated GCF cytokines[9],[10], only few studies have compared the cytokine profiles in blood of GAgP patients.[11],[12] Thus this study was conducted for evaluating the level of selected pro and anti-inflammatory cytokines in GCF and peripheral blood of GAgP patients as compared to periodontally healthy individuals.
Considering the clinical parameters, at baseline, the difference in the GI, PPD and CAL were found to be significantly high for Group A, than Group B, whereas PI between both the groups was not statistically significant. This could be explained as the characteristic feature of aggressive periodontitis is that the amount of plaque present in patients is not concomitant with the amount of periodontal destruction.[2]
At 6 month post surgical therapy a statistically significant reduction was seen in all the clinical parameters in Group A, similar to the findings of Toker et al[9] and Oliveira et al[10] who observed a significant decrease in GI, PPD and CAL post periodontal therapy in GAgP patients, thus suggesting good patient compliance with periodontal maintenance at regular intervals.
When the level of cytokines was assessed at baseline in Group A, IL-1β was significantly higher amongst all the proinflammatory cytokines, in agreement with the findings of Vahabi et al[13], could be because it is an important mediator of inflammation.[14]
Among the anti-inflammatory cytokines in Group A at baseline, our study revealed a higher level of IL-10. Lappin et al[15] suggested a high increase in the number of inflammatory leukocytes that express the anti-inflammatory cytokines in aggressive periodontitis than chronic periodontitis. However its level was not comparable with that of IL-1β, thus could explain the massive periodontal destruction seen in GAgP subjects. At 6 months after periodontal surgery, statistically significant reduction was observed in the concentration of IL-1β, IL-2 and GM-CSF with a simultaneous but comparatively less decrease in the level of IL-6 and TNF-α, comparing favorably with the studies of Thunell et al[16] and Yue et al[17]. Teles et al[18] demonstrated higher level of GM-CSF in GAgP patients than in periodontally healthy subjects. The effect of GM-CSF on osteoclast is controversial, both inhibition and stimulation are reported.[19] There is paucity of literature regarding the role of GM-CSF in aggressive periodontitis, but from our observations it can be inferred that its reduction as a result of therapy indicates that this cytokine may play a role in pathogenesis of GAgP.
The decrease in the levels of anti-inflammatory cytokines, IL-10 and IL-4 among Group A were found to be significant at 6 month post therapy, analogous to the findings of Gamonal et al.[20] This reduction could be due to a concomitant reduction of proinflammatory cytokines observed following periodontal therapy thereby maintaining a balance.
When the level of cytokines in Group Awere compared to Group B at baseline, among the proinflammatory cytokines significantly higher levels were seen for both IL-1β and IL-2 in Group A than TNF-α, IL-6 and GM-CSF, similar to the findings of Kurtis et al[21] and Bastos et al.[22] This raised level of IL-1β and IL-2 could denote their significant role in pathologic process of the inflammatory response in aggressive periodontitis.
Significantly higher levels of IL-4 and IL-10 were seen in Group A than Group B at baseline. Contrary to our findings Giannopoulou et al[23] reported higher level of IL-4 in GCF from healthy subjects when compared to AgP individuals. At 6 months after surgery the difference in the levels of IL-2, IL-6, TNF-α, IL-4, IL-10 and GM-CSF were not significant between the two groups. This shows that the levels of all the cytokines are approaching the levels in periodontally healthy individuals.
When the level of cytokines was assessed in blood at baseline in Group A, highest level of IL-6 was observed amongst all the proinflammatory cytokines in agreement with the finding by Robati et al.[24] In Group A among the anti-inflammatory cytokines high level of IL-4 were observed, similar to the findings of McFarlane et al.[25] However very few studies have evaluated IL-4 level in the blood. From baseline to 6 months after periodontal surgical therapy, there was significantly higher reduction in the level of IL-6 along with reduction in 1β, IL-2, IL-6, TNF-α and IL-10. IL-4 and GM-CSF did not show any significant difference as observed by Sigusch et al.[26]
When compared between Group A and Group B at baseline, the levels of proinflammatory cytokines in blood: IL-1β, IL-6, and IL-2, were found to be highly raised in Group A. The difference seen in the anti-inflammatory cytokines in Group A were statistically significant only for IL-10, whereas for other cytokines TNF-α, IL-4 and GM-CSF there was no significant rise in the level when compared with control group, contrary to Chen et al[27] with very low levels of serum cytokines IL-1β, IL-2 in patients with chronic periodontitis.The insignificant difference for TNF-α as observed could be because of the role of its soluble receptor which acts as anti inflammatory cytokine.[28] IL-10 has been reported to be the most abundantly expressed cytokine in periodontitis granulation tissue[29], therefore, spread of this cytokine to the circulation is a possibility. IL-4 has been shown to induce apoptosis in lipopolysaccharide, its reduction may result in a breakdown of the regulation of immune function and a subsequent elevated secretion of bone resorbing mediators.[30],[31] At 6 months after periodontal surgical therapy, the level of IL-1β could not approach the level of Group B. However no significant difference wasseen for rest of the cytokines between both groups.
It has not been fully elucidated as to how much the local inflammatory response in the periodontium can influence the systemic levels of inflammatory mediators as overall level in blood of cytokines were not raised to the levels found in GCF. The gastro intestinal tract normally contains a population of nonpathogenic flora with a continuous production of endotoxins in their outer membrane within the gut.[32] Thus it can be hypothesized that the cytokines produced by these microorganisms could contribute to their raised level in blood.
Conclusion
Within the limitations of our study the results indicate that the levels of cytokines were elevated in GCF of subjects with GAgP and reduced significantly 6 months post therapy accompanied by significant improvement in clinical parameters. Although peripheral blood cytokines levels were higher as compared to healthy individuals but the difference was not as significant as seen in GCF. Thus it could be inferred that severe periodontal destruction seen in GAgP is due to production of virulence factors by pathogenic bacteria in a more concentrated environment. The high level of blood cytokine could be because of the systemic endotoxin produced by gut flora along with the cytokines produced by periopathogens. Further large-scale studies are needed to determine the impact of periodontal disease on systemic inflammation.
Referances
1. Armitage G. Development of a classification system for periodontal diseases and conditions. Ann Periodontol, 1999; 4; 1:1-6.
2. Lang N, Barthold PM, Cullinan M, Jeffcoat M, Mombelli A, Murakam S, Page, R,Papapanou P, Tonetti M & Van Dyke T. Consensus Report: Aggressive periodontitis. Annals of Periodontology, 1999; 4: 53.
3. Meng H, Xu L, Li Q, Han J, Zhao Y. Determinants of host susceptibility in aggressive periodontitis. Periodontol 2000 2007; 43: 133-159
4. Page RC, Offenbacher S, Schroeder, Seymour GJ and Kornman KS.Advances in pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontology 2000, 1997; 14: 216–248.
5. Tonetti M. S. & Mombelli A. Early onset periodontitis. Annal of Periodontology 1999; 4: 39–52.
6. Takeuchi Y, Umeda M, Ishizuka M, Huang Y, Ishikawa I.Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Japanesepopulation. J Periodontol. 2003; 74:1460-1469.
7. Malamud D. Salivary diagnostics - The future is now. J Am Dent Assoc 2006; 137: 284-286.
8. Donnelly RE Fenton MI, Finbloom DS, Gerrard TL. Differential regulation of IL-1 production in human monocytes by IFN-y and IL-4. J Immunol 1990 145: 569-575.
9. Toker H, Poyraz O, Eren K. Effect of periodontal treatment on IL-1β, IL-ra, and IL-10 levels in gingival crevicular fluid in patients with aggressive periodontitis. J Clin Periodontol 2008; 35: 507-513.
10. Oliveira APL, Faveri M, Gursky LC, Mestnik MJ, Feres M, Haffajee AD et al. Effect of periodontal therapy on GCF cytokines in generalized aggressive periodontitis subjects. J Clin Periodontol 2012; 39: 295-302.
11. Yamazaki K, Honda T, Oda T, Ueki-Maruyama K, Nakajima T, Yoshie H, Seymour GJ. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodont Res 2005; 40: 53–58.
12. Duarte PM, Rocha M, Sampaio E, Mestnik MJ, Feres M, Figueredo LC, Bastos MF, and Faveri M. Serum levels of cytokines in subjects with Generalized chronic and Aggressive periodontitis before and after non- surgical periodontal therapy: A pilot study. J Periodontol 2010; 81: 1056-1063.
13. Vahabi S, Sattari M, Taheraslani M, Bagheban A. Correlation between Interleukin-1β, Interleukin-6 and Tumor Necrosis Factor-α and Clinical Parameters in Chronic and Aggressive Periodontal Disease. J Periodontol Implant Dent 2011; 3: 51–56.
14. Dinarello, C. A. Interleukin-l and Its Biologically Related Cytokines. In: Lymphokines and the Immune Response. pp. 145-179. (S. Cohen, Ed.) CRC Press, Boca Raton, FL (1990).
15. Lappin DF, Macleod CP, Kerr A, Mitchell T, Kinane DF. Anti-inflammatory cytokine IL-10 and T cell cytokine profile in periodontitis granulation tissue. Clincal Experimental Immunology 2001; 123: 294-300.
16. Thunell DH, Tymkiw KD, Johnson GK, Joly S, Burnell KK, Cavanaugh JE, Brogden KA, Guthmiller JM. A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J Periodontal Res. 2010; 45: 148-152.
17. Yue Y, Liu Q, Xu C, Ty Loo W, Wang M, Wen G, Nb Cheung M, Bai LJ, Dou YD, Wc Chow L, Hao L, Tian Y, Li JL, Ys Yip A, Ly Ng E. Comparative evaluation of cytokines in gingival crevicular fluid and saliva of patients with aggressive periodontitis. Int J Biol Markers. 2013; 28: 108-112.
18. Teles RP, Gursky LC, Faveri M, Rosa EA, Teles FRF, Feres M, Socransky SS, Haffajee AD. Relationship between subgingival microbiota and GCF biomarkers in generalized aggressive periodontitis. J Clin Periodontol 2010; 37: 313-323.
19. Beutler, B. and A. Cerami: Cachectin (Tumor Necrosis Factor) and Lymphotoxin as Primary Mediators of Tissue Catabolism, Inflammation, and Shock. In: Lymphokines and the Immune Response. pp. 199-212. (S. Cohen, Ed.) CRC Press, Boca Raton, FL (1990).
20. Gamonal J, Sanz M, O’Connor A, Acevedo A, Martinez B & Silva A. Delayed neutrophil apoptosis in chronic periodontitis patients. Journal of Clinical Periodontology 2003; 30: 616-623.
21. Kurtis¸ B, Tuter G, Serdar M et al. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha in patients with chronic and aggressive periodontitis. J Periodontol 2005; 76: 1849–1855.
22. Bastos MF, Lima JA, Vieira PM, Mestnik MJ, Faveri M, Duarte PM.TNF-α and IL-4 levels in aggressive periodontitis subjects. Oral Diseases 2008; 15: 82-87.
23. Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol 2003; 30: 145–153.
24. Robati M, Ranjbari A, Boroujerdnia MG, Chinipardaz Z. Detection of IL-4, IL-6 and IL- 12. Serum Levels in Generalized Aggressive Periodontitis. Iran J Immunol 2011; 8: 3.
25. McFarlane CG, Meikle MC. Interleukin-2, interleukin-2 receptor and interleukin-4 levels are elevated in the sera of patients with periodontal disease. J Periodontal Res. 1991; 26: 402-408.
26. Sigusch B, Klinger G, Glockmann E and Simon HT. Early-Onset and Adult Periodontitis Associated With Abnormal Cytokine Production by Activated Lymphocytes. J Periodontol 1998; 69: 1098-1104.
27. Chen CC, Chang KL, Huang JF, Huang JS, Tsai CC. Kaohsiung J Med Sci. Correlation of interleukin-1 beta, interleukin-6, and periodontitis. 1997; 13: 609-617.
28. Graves DT, Delima AJ, Assuma R, et al. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontol 1998; 69: 1419.
29. Kim HO, Kim HS, Youn JC, Shin EC and Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. Journal of Translational Medicine 2011; 9: 113.
30. Shapira L, Van Dyke T E & Hart T C. A localized absence of interleukin-4 triggers periodontal disease activity: a novel hypothesis. Medical Hypotheses 1992; 39: 319–322.
31. Yamamoto M, Kawabata K, Fujihashi K et al. Absence of exogenous interleukin- 4-induced apoptosis of gingival macrophages may contribute to chronic inflammation in periodontal diseases. Am J Pathol 1996; 148: 331–339.
32. Leeuwen VP, Boermeester M, Houdijk A, Ferwerda Ch, Cuesta M, Meyer S and Wesdorp R. Clinical significance of translocation. Gut 1994:28-34.
|