Introduction
Squamous cell carcinoma of the oral cavity is the most common cancer of the head neck region.[1] Major modalities used in treating cancer are: surgical resection, radiation therapy and chemotherapy and combination of these procedures. Surgery and radiation therapy are considered local or definitive therapies, but do not address the issue of distant metastasis which can only be achieved with chemotherapy.[2] The treatment of patients with carcinoma of oral cavity is principally directed at controlling the primary tumor and regional neck metastases.[3] Neck dissection is a frequent part of treatment of oral cancer.[4] Overall, the management of cancer of the oral cavity is evolving rapidly and a multidisciplinary approach to the patient who has oral cavity cancer is important to ensure the highest quality of patient care.[5] Management of the N0 neck has been debated extensively. This article presents the controversies surrounding management of the N0 neck, and a case report of oral squamous cell carcinoma with clinically negative neck .
Case Report
A 33 year-old man reported with a painless, exophytic lesion over his left posterior buccal mucosa. The lesion was first noticed by the patient 4 weeks earlier and it gradually increased in size. Personal history revealed the history of betel quid chewing, smoking and alcohol abuse for several years. There was no family history of malignancy. Medical history of the patient had no important episodes. The intraoral examination revealed an irregular, diffuse, reddish exophytic lesion over his left posterior buccal mucosa opposite molars and extending up to retromolar trigone, about 3 × 2 cm in size, oval in shape, non-tender on palpation and firm in consistency (Fig 1). Extra oral examination revealed no lymphadenopathy in submandibular or other neck triangles. Radiographic examination showed no bony invasion. Under the impression of malignancy, an incisional biopsy was performed under local anesthesia on the same day and the specimen was submitted for histopathological examination, which demonstrated well differentiated squamous cell carcinoma. After obtaining the results of blood investigations with no abnormality and a fitness for surgery a written consent was taken from the patient. Wide excision of lesion with 2cm safe margin and marginal resection of mandible was performed under GA. Buccal fat pad was used as a graft in the surgical defect (Fig 3). Along with it a supraomohyoid neck dissection as a preventive measure against micro metastasis was performed. While performing the neck dissection levels I II, III lymph nodes were removed (Fig 2). Hemostasis was achieved and wound closed in layers. The specimen (Fig 4, 5) was sent for histopathological examination and a well differentiated squamous cell carcinoma was reported. Surgical margins were free and no metastasis was found in lymph nodes. The patient was followed up for two years with no recurrence noted till date (Fig 6).
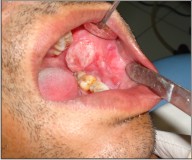 | Fig 1 : Pre-operative Photograph Showing Intra-oral Lesion.
 |
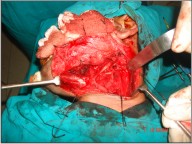 | Fig 2 : Intra-operative Photograph Showing Supraomohyoid Neck Dissection.
 |
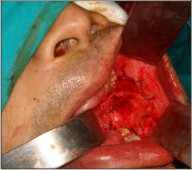 | Fig 3 : Intra-operative Photograph Showing Buccal Fat Pad Used As A Graft In The Surgical Defect.
 |
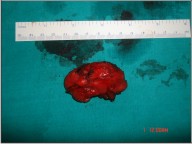 | Fig 4 : Surgical Specimen Of The Excised Lesion.
 |
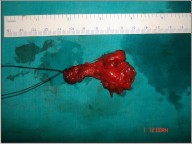 | Fig 5 : Surgical Specimen Of Supraomohyoid Neck Dissection.
 |
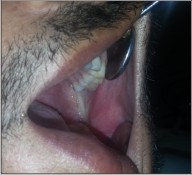 | Fig 6 : Post – Operative Photograph.
 |
Discussion
Squamous cell carcinoma of the oral cavity can be defined as an invasive epithelial neoplasm with varying degrees of squamous differentiation and propensity to early and extensive lymph node metastasis, occurring predominantly in alcohol and tobacco using adults in 5th and 6th decades of life.[6] Oral cancer ranks number one among men and number three among women in India .Buccal mucosa is the most common site for oral cancer in Indian population.[7] When an oral mucosal lesion is encountered that is suspicious in nature or is of questionable origin, an effort should be made to understand the underlying pathology. Clinical presentations that warrant immediate action to rule out squamous cell carcinoma include non-healing ulceration or unexplained swelling of approximately 3 weeks duration and all red or all white lesions. In these cases a biopsy is mandatory with definitive diagnosis by histologic examination. Additionally it is recommended that tooth mobility unrelated to periodontal disease receive thorough investigation. Fine needle aspiration biopsy (FNAB) can facilitate the diagnosis of head and neck cancer in the patients presenting with cervical lymphadenopathy. If there is any question as to whether cervical lymph nodes are pathologically enlarged, or if previous FNABs have yielded equivocal or non-diagnostic results, ultrasound or computed tomography (CT) guided FNAB are useful alternatives to confirm the diagnosis.[8] The factors that influence the choice of initial treatment are tumor factor, patient factor and physician factor.[9],[10] The ultimate goals in treatment of cancer of the oral cavity are to eradicate the cancer , preserve or restore form and function , minimize the sequelae of treatment , and prevent subsequent new primary tumors.[10] Small cancers of the oral cavity are usually managed by surgery alone and larger cancers are usually treated with primary surgery followed by chemoradiation.[4] Treatment of regional lymphatic tissue is an integral component in the management of squamous cell carcinoma of the oral cavity. More than 50% of patients with squamous cell carcinoma of the oral cavity have lymph node metastasis and histological confirmation of metastasis disease is the most important prognostic factor .The cure rates drop to nearly half with involvement of regional lymph nodes.[11] In general terms, lymph nodes in levels I-III are considered sentinel for oral cavity cancers. In this sense, these are the first lymph nodes expected to contain metastatic squamous cell carcinoma when the neck in fact contains cancer. This well accepted concept forms the basis for prophylactic neck dissections where the likelihood of occult neck disease exceeds 20%.[12] Paramount among the challenges and controversies facing the head and neck surgeon is the proper treatment of the No neck. There is great controversy regarding the optimal therapy for clinically negative necks .Three treatment options are available for management of No neck: observation, elective neck irradiation (ENI) and elective neck dissection (END). The proponents of observation cite the morbidity of END as a reason to observe. Another argument for close observation is that with close follow up; any cervical metastasis can be detected early and then treated with adequate therapy. Moreover the occult metastatic rate to the neck from oral cavity cancer is 34%. Hence, it is argued that nearly two thirds of the patients would be exposed to the morbidity of a neck dissection unnecessarily.[13] Weiss et al [14] created a decision tree analysis and concluded that observation is the preferred option when the probability of occult metastasis is less than 20% and elective neck treatment (irradiation or dissection) is preferred if the probability of occult metastasis is greater than 20%. In oral squamous cell carcinoma the sites with a less than 20% occult metastatic rate to the neck are those associated with T1/T2 lip carcinomas, T1/T2 oral tongue carcinomas that are less than 4mm thick, T1/T2 floor of mouth cancers less than or equal to 1.5 mm thick. Hence, “wait and watch” approach can be adopted in these patients. Once the decision to treat the No neck has been made, there are two possible treatment modalities, elective neck irradiation ENI and elective neck dissection END. The modality that is chosen to treat the primary cancer may also help in formulating a decision as to how to treat the neck. If primary radiation therapy is used, ENI can be performed .If the neck is going to be entered to remove the primary tumor, an END can be performed. Literature provides no clear cut recommendation for using ENI or END to treat No necks. The most important factors in guiding this decision should be the patient’s informed decision, physician and institution experience, risk of second primary occurrence in the future, and the modality chosen to treat the primary cancer.[13] In the last decade, emphasis has been placed on the use selective neck dissection (SND) in the management of No necks. This procedure , initially developed as a staging operation to assess the presence of occult metastasis, has evolved into another treatment modality for node negative necks .The use of SND in clinically No patients with oral cancer has resulted in excellent control disease with recurrence rates of less than 10%[15]. Clinically, SND has been most utilized for elective treatment of regional lymphatics when the risk of metastasis is high. Results of this strategy indicate that regional control and survival rates are similar to those of more extensive neck dissections. There is substantial evidence that micro metastases cannot be detected at present by non-invasive methods so it is essential to dissect the neck in order to reduce the risk of regional recurrence and its related mortality. SND thus also serves as a staging procedure and can be used for decision making regarding the need for adjuvant post-operative radiation therapy. This type of surgical dissection appears to be supported by pathological, sub pathological and clinical data. Even microscopic extra capsular spread is of critical importance and must be sought especially in small volume metastatic disease.[16] Present literature suggests SND (I-III) or Supraomohyoid neck dissection is the ideal solution to the dilemma for surgeons as to how to properly manage the N0 neck. A sufficient body of literature supports the performance of elective neck dissection for T1N0 and T2N0 squamous cell carcinoma of oral cavity, identifying the incidence of occult neck disease in these cases of 36% to 42% .[12] Radiotherapy used either pre or post neck dissection has been shown to reduce the incidence of neck failure by at least 50% for all N stages .Moreover the fact that pre or post radiotherapy makes no difference in the outcome has encouraged many surgeons to use radiotherapy as a postoperative adjunct, as this consequently eases the dissection and reduces the rate of complications .The presence of large primary tumor and the presence of positive margins normally dictate the use of radiotherapy. Usually patients with N0 neck do not require radiotherapy. On the other hand radiotherapy has been shown to be beneficial in the presence of multiple node metastasis and ECS.[15] Although the decision to observe or treat the N0 neck is left to the choice of the patient and the head and neck oncologist, in oral cavity carcinoma the only clinically N0 necks for which observation is appropriate are those associated with T1/T2 lip carcinomas, T1/T2 oral tongue carcinomas that are less than 4mm thick, T1/T2 floor of mouth cancers less than or equal to 1.5 mm thick.[13]. The nature and stage of the cancer, risk of local and distant recurrence, and comorbidity conditions must be assessed when designing an optimal treatment for patients. Aggressive therapy is not for everyone, and many patients will not tolerate chemotherapy and radiation. It is through ongoing randomized clinical trials that most appropriate therapies will emerge.
References
1. Arduino PG, Chiecchio A, Tirone F, Bertolusso G. Clinical and histopathologic independent prognostic factors in oral squamous cell carcinoma: a retrospective study of 334 cases. J Oral Maxillofac Surg 2008; 66: 1570-1579.
2. Haddad R, Annino D, Tishler RB. Multidisciplinary approach to cancer treatment: focus on head and neck cancer. Dent Clin N Am 2008; 52:1-17.
3. Cunninghum MJ, Johnson JT, Myers EN, Schramm VL, Thearle RN. Cervical lymph node metastasis after local excision of early squamous cell carcinoma of the oral cavity. Am J Surg 1986; 152:361-366.
4. Campana JP, Meyers AD. The surgical management of oral cancer. Otolaryngol Clin N Am 2006; 39:331-348.
5. Ballonoff A, Chen C, Raben D. Current radiation therapy management issues in oral cavity cancer. Otolaryngol Clin N Am 2006; 39:365-380.
6. Johnson N, Franceschi S, Ferlay J, Ramadas K, Schmid S, MacDonald DG, et al. Squamous cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. IARC Press Lyon, 2005, 168-175.
7. Sankarnarayanan R. Oral cancer in India: an epidemiologic and clinical review. Oral Surg Oral Med Oral Pathol 1990; 69: 325- 330.
8. Noonan VL, Kabani S. Diagnosis and management of suspicious lesions of the oral cavity. Otolaryngol Clin N Am 2005; 38:21-35.
9. Breau RL, Suen JY. Management of the N0 neck. Otolaryngol Clin N Am 1998; 31:657-669.
10. Shah JP. Surgical approaches to the oral cavity primary and neck. Int J Radiation Oncology Biol Phys 2007; 69:S15-S18.
11. Shah JP, Anderson PE. Evolving role of modifications in neck dissection for oral squamous carcinoma .Br J Oral Maxillofac Surg 1995; 33:3-8.
12. Carlson ER, Cheung A, Smith B, Pfohl C. Neck dissection for Oral / Head and neck cancer. J Oral Maxillofac Surg 2006; 64:4-11.
13. Jalisi S .Management of the clinically negative neck in early squamous cell carcinoma of the oral cavity. Otolaryngol Clin N Am 2005; 38:37-46.
14. Weiss MH,Harrison LB, Issacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 1994;120:699-702.
15. Chummun S, McLean NR, Ragbir M. Surgical education: neck dissection. The British Association of Plastic Surgeons 2004; 57: 610-623.
16. Ferlito A, Rinaldo A, Robbins KT, Leemans CR, Shah JP, Shaha AR, et al. Changing concepts in the surgical management of the cervical node metastasis. Oral Oncology 2003; 39: 429-435.
|