Introduction
Micro-organisms are ubiquitous. Although most microorganisms are beneficial & necessary for human well-being, microbial activities may have undesirable consequences, such as food spoilage & disease.[1]
It is essential to be able to kill or inhibit their growth to minimize their destructive effects.
Goal is :
(1) To destroy pathogens & prevent their transmission.
(2) To reduce or eliminate microorganisms responsible for the contamination.[2]
Dental patients & DHCP can be exposed to pathogenic microorganisms including cytomegalovirus (CMV), HBV, HCV, herpes simplex virus types 1 and 2, HIV, Mycobacterium tuberculosis, staphylococci, streptococci, & other viruses & bacteria that colonize or infect the oral cavity & respiratory tract. [6]
Failure to properly disinfect or sterilize equipment carries not only risk associated with breach of host barriers but also risk for person-to-person transmission (e.g., hepatitis B virus) and transmission of environmental pathogens (e.g., Pseudomonas aeruginosa).[7]
The diseases may be transmitted from the patient to the dentist, or any other person involved in dental care procedure including dental surgery assistant or even the dental laboratory personnel.[10]
Routes Of Disease Transmission
Organisms can be transmitted in dental settings through :
1) Direct Contact with blood, oral fluids, or other patient materials
2) Indirect Contact with contaminated objects (e.g., instruments, equipment, or environmental surfaces)
3) Contact of conjunctival, nasal, or oral mucosa with droplets (e.g., spatter) containing microorganisms generated from an infected person & propelled a short distance (e.g., by coughing, sneezing, or talking)
4) Inhalation of airborne micro organisms that can remain suspended in the air for long periods.[3],[6]
Image 1 : Routes of Cross-contamination & Cross-infection in Dental setting.
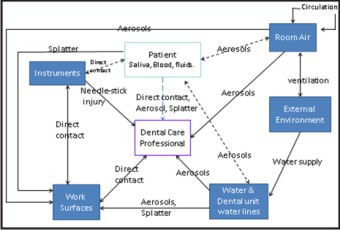 | Image 1 : Routes of Cross-contamination & Cross-infection in Dental setting.
 |
Chain Of Infection:
The six components in the chain of infection include the infectious agent, a reservoir, a portal of exit, a means of transmission, a portal of entry, and a susceptible host.[4] Dental impressions, maxiliomandibular registration bases and apparatus, trial and final prostheses are all exposed to contamination in the patient's mouth. The maintenance of asepsis is essential to prevent the transmission of bacteria, viruses, and fungi. It is especially important when immediate dentures, obturators, and implants are fitted, because the operative sites are highly vascular wounds.[23]
Image 2 : Preventive measures - To break the " Chain of infection".
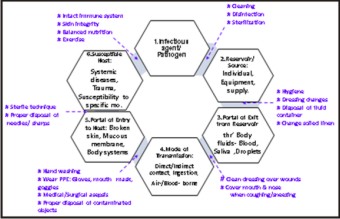 | Image 2 : Preventive measures - To break the " Chain of infection".
 |
Classification Of Level Of Risk
Based on article in DCNA 1996 there are 5 classes of risk in dental health care professionals. The classes are based on the level of risk and ease of prevention.[16]
Table 1 : Classification of Disease risk & its Prevention in Dental healthcare professionals.
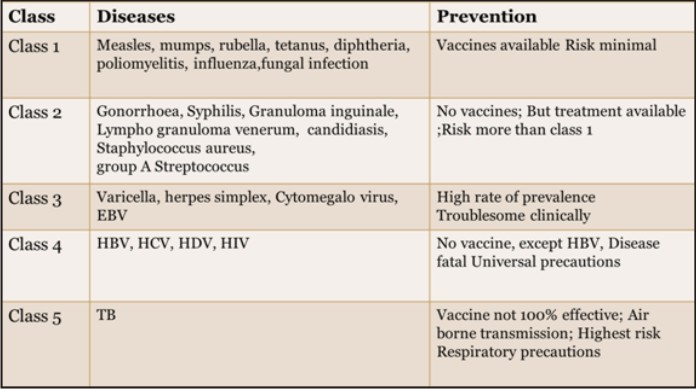 | Table 1 : Classification of Disease risk & its Prevention in Dental healthcare professionals.
 |
A Rational Approach To Disinfection And Sterilization:
Earle H. Spaulding classified the nature of disinfection of instruments & items for patient care according to the degree of risk for infection involved in use of the items asà
A) Critical - High risk for infection if they are contaminated with any microorganism.
Critical instruments include forceps, scalpels, bone chisels, scalers and surgical burs.
They should be sterilized after each use, by steam under pressure (autoclaving), dry heat, or heat/chemical vapor.
B) Semi-critical - Contact mucous membranes or non-intact skin.
Instruments such as mirrors, reusable impression trays and amalgam condensers.
Should be sterilized after each use. In some cases high level disinfectant may be used.
C) Non-critical – That come into contact only with intact skin,
Such as external components of x-ray heads, blood pressure cuffs and pulse oximeters.
Reprocessed between patients by intermediate-level or low-level disinfection.[7]
A 1991 study expanded the Spaulding scheme by dividing the noncritical environmental surfaces into housekeeping surfaces and medical equipment surfaces.[19]
CDC has divided noncritical surfaces in dental offices into -
A) Clinical contact surfaces – directly contaminated by patient materials either by direct spray or spatter generated during dental procedures or by contact with DHCP’s gloved hands.
B) Housekeeping surfaces - e.g. floor, sinks & walls.[7]
Infection Control
Infection Control is based upon the principle that transmission of infectious diseases will be prevented when any of the steps in the chain are broken or interrupted. Emphasis is paid not only to the patient protection but also to all members of the dental team.
Infection Control Procedures Involve :
1) Patient Screening
2) Personal hygiene
3) Personal protection
4) Instrument processing
5) Surface asepsis
6) Patient treatment
7) Laboratory disinfection [23]
Sterilisation & Disinfection of patient care instruments & material used are part of Infection control protocol in health care setting including dental care.[9]
Patient Screening
Any treatment is performed only after a comprehensive patient evaluation. Dentist’s review of the patient’s medical history is mandatory at the onset of every clinical appointment.[17]
Personal Hygiene
Thorough forearm and hand washing is mandatory before and after treatment. Fingernails are kept clean and short to prevent perforation of gloves and accumulation of debris. Fingernail polish is not worn. Use surgical head cap , face mask & eye protecting glasses & long sleeved clinical coats. [6],[12]
Personal Protection
Gloves are worn at all times when treating patients. Masks are worn in the patient treatment area and when the dentist is manipulating the prostheses in the laboratory.
In recent literature, attention has been focused on the inhibitory effects of certain latex gloves on the setting times of poly(vinyl siloxane) putty impression material. Consequently, it has been recommended that gloves should not be worn
when dispensing, mixing, or handling poly(vinyl siloxane) putty materials. However, it is better to wear synthetic gloves in place of or over latex gloves to avoid disruption of the barrier technique when such materials are used.[23]
In June 1982, the council on dental therapeutics adopted a resolution recommending that all dental personnel having patient contact including dentists, dental students and dental auxiliary personnel, and all dental laboratory personnel receive the Hepatitis B vaccine. Residents are required to have current immunizations against communicable diseases, including hepatitis B. [18]
Vaccination programme considered the most effective cross-infection control measure to protect dental personnel, and in turn their patients, from a potentially fatal disease.[23]
Instrument Processing & Surface Asepsis
Processing Instruments :
All critical & semi-critical dental instruments that are heat stable should be sterilized after each use by steam under pressure (autoclaving), dry heat, or chemical vapor.
Before sterilization or high-level disinfection, instruments should be cleaned so that any debris is removed.
Heavy-duty gloves should be worn when handling contaminated instruments.
Instruments should soak in water or disinfectant/detergent as soon as possible after use to prevent drying of debris. Instrument cassettes and mechanical cleaning (e.g., ultrasonic cleaners) may be used to reduce direct handling of contaminated instruments.
Packaging rinsed and dried instruments before sterilization protects them from contamination after they are removed from the sterilizer and during transport chairside or to storage.
Sterilization is recommended for all high-speed dental handpieces, low-speed handpiece components used intraorally and reusable prophylaxis angles. [5]
Table 2 : Instrument Processing Table[5] :
![Table 2 : Instrument Processing Table[5] :](article-image-4176-TABLE_2_INSTRUMENT_PROCESSING_TABLE[5]_.jpg) | Table 2 : Instrument Processing Table[5] :
![Table 2 : Instrument Processing Table[5] :](images/article-image-enlarge.jpg) |
Disinfection of HBV-, HCV-, HIV- or TB-Contaminated Devices:
The CDC recommendation for high-level disinfection of HBV-, HCV-, HIV- or TB-contaminated devices is appropriate because experiments have demonstrated the effectiveness of high-level disinfectants to inactivate these and other pathogens that might contaminate semi critical devices.[7]
Surface Disinfection :
Non critical medical equipment surfaces should be disinfected with an EPA-registered low- or intermediate-level disinfectant. Currently, some EPA-registered disinfectants have contact times of one to three minutes.[7]
According to Miller and Palenik in 1994 chemicals used for surface and equipment asepsis are -
# Chlorine – e.g. sodium hypo chlorite.
# Phenolic compounds.
- Water based – Water with ortho – phenyl phenol or Tertiary amylphenol or O benzyl – p – chlorophenol
- Alcohol based – Ethyl or iso propyl alcohol with O phenyl phenol or Tertiary amylphenol
# Iodophor – butoxypoly propoxy poly ethoxy ethanol iodine Complex.[10]
Factors affecting the efficacy of both disinfection & sterilization:
a) Prior cleaning of the object.
b) Organic and inorganic load present.
c) Type and level of microbial contamination.
d) Concentration of and exposure time to the germicide.
e) Physical nature of the object (e.g., crevices, hinges, and lumens).
f) Presence of biofilms.
g) Temperature and pH of the disinfection process.
h) Relative humidity of the sterilization process (e.g., ethylene oxide).[1],[7]
Treating Prosthodontic Patients In The Clinic:
The use of a strict system of zoning in the clinic will reduce the number of areas contaminated and thereby facilitate maintenance of asepsis.[23]
Before seating the patient the operatory and chair is cleaned and wiped with a disinfectant solution, the area is sprayed and left for a minimum 10 minutes. The BDA recommends 70% isopropyl alcohol, hypochlorite solution (containing 1% available chlorine), or 2% glutraldehyde solution for disinfecting contaminated surfaces under different circumstances.The dental chair is covered with a disposable plastic sheath, which is removed after treatment.[24] All patients rinse with chlorhexidine gluconate 0.12% before treatment.[25] Patients wear protective eye wear. Hands are washed with an antimicrobial cleanser before gloving. Once gloved, only the patient and barrier covered areas or areas that have been cleaned and disinfected are touched. A unit dose concept may be adopted for use in the clinic as a cross-contamination control measure. This may be applied to many items and materials in prosthodontics, such as impression materials and waxes. The materials should be dispensed by a non-contaminated assistant prior to patient contact. This will eliminate the possibility of cross-contamination occurring via containers, tubes, and dispensers.[26]
Large, non-sterilizable items used in the operatory, such as impression material dispensing guns, articulators, face bows, water bath, silicone spray bottles, tooth shade, and mold guides are disinfected by wiping, spraying, or immersion with the appropriate disinfectant solution.
Disinfectants can be used to decontaminate impressions, occlusion rims, diagnostic casts, and trial and final prostheses (including immediate-replacement dentures, partial denture frameworks, acrylic resin dentures, and obturator).The most acceptable disinfectant is 2% buffered glutaral dehyde solution for minimum immersion time of 10 minutes. Tuberculosis is transmitted by sputum & is consequently a high risk in a prosthodontic practice. This is especially true when treating older patients, who are particularly vulnerable to infection. To protect against the transmission of M tuberculosis, a routine disinfection of 1 hour in glutaraldehyde solution would be necessary.[23]
All items leaving the clinic that are used in direct patient care or touched during patient care procedures that cannot be subjected to sterilization procedures are disinfected or placed in the phenol disinfection solution within a sealed plastic bag for 10 minutes before departure. New latex gloves are worn for the disinfection procedures. Metal impression trays are hanged and autoclaved before use. Adhesives for impression trays are used in individual dose quantities to prevent cross-contamination. Polyvinyl siloxane, polysulfide, impression compound, and ZOE impression materials are thoroughly rinsed under water and immersed in a 5.25% sodium hypochlorite solution for 10 minutes.[27],[28] 2% glutaraldehyde did not affect the accuracy and dimensional stability of polyether and polyvinyl siloxane impression materials after immersion for 30 or 60 minutes.[29] Alginate and polyether impressions are rinsed under water, sprayed with a 5.25% sodium hypochlorite solution and sealed in a plastic bag for at least 10 minutes.[30]
Wax, ZOE, and resin centric relation records are rinsed under water and sprayed with a 5.25% sodium hypochlorite solution and placed in a plastic bag for 10minutes.[31] 20-minute immersion in 2% ID 210 solution (Durr Dental, Bissingen, Germany )has no adverse effects on the dimensional stability or surface detail reproduction of rigid material such as an impression compound, impression piaster, and zinc oxide-eugenol impression material.[32] Stone casts requiring disinfection are sprayed with a 5.25% sodium hypochlorite solution for at least 10 minutes. Complete dentures and provisional restoration are immersed in a 5.25% sodium hypochlorite solution for 10 minutes. Or Rinsed under running water, 4% chlorhexidine scrub for 15 seconds followed by a 3 minutes contact time with chlorine dioxide. Also can be Sterilized by ethylene oxide gas-450-800 mg/I.Removable partial dentures with metal bases are sprayed with 2% glutaraldehyde solution and held in a plastic bag for 10 minutes.
Laboratory Norms:
The dental laboratory becomes the second line of infection control barriers that protect the patients, residents, assistants, and faculty. All prostheses that enter and leave the laboratory are disinfected. Within the laboratory the prosthesis of each patient is kept separate through barrier, disinfection, and sterilization systems. Before impressions are poured in the laboratory, hydrophilic impression materials are sprayed with a sodium hypochlorite solution (5.25%) and placed in a plastic bag for a minimum time of 1 minute. Hydrophobic impression materials are immersed in disinfectant solution for 10 minutes. All prostheses entering the laboratory are scrubbed with disinfectant solution. Those leaving the laboratory are immersed in a 5.25% sodium hypochlorite solution for a minimum of 10 minutes. New gloves are worn in the laboratory area for grinding and polishing procedures, protective clothing is worn in the laboratory and discarded before the dentist leaves the laboratory area.
Laboratory countertops are cleaned and wiped with disinfectant solution at the end of each day.
Individually packaged chemiclaved laboratory burs are available in the laboratory. After the desired procedure is accomplished, the laboratory bur is cleaned and placed in a new bag for sterilization. The burs are used for one patient only and then re sterilized.For polishing the lathe, when the technician should use Individually packaged sterile polishing wheels, designated for use with pumice. The addition of an antiseptic product that contained Octenidine as active agent to conventional pumice reduced the number of microorganisms by 99.999%. The mix of steribim with water reduced the number of bacteria by 99%. The wheel is wet with water to soften it before use.[33] If prosthesis becomes contaminated during laboratory procedures, it is disinfected and the laboratory procedure continued.
Disinfectant soaks (eg, 3% hexachlor ophene, 5% sodium hypochlorite, or 2% giutaraidehyde solution) have been suggested for use on contaminated prostheses before polishing. The use of an ultrasonic bath will enhance tbe biocidal activity of the disinfectant.[34]
Final polish is accomplished using a sterile wheel with non -contaminated acryluster. The acryluster is applied to the sterile wheel once before polishing to eliminate cross-contamination.Clean-up involves disposal of the plastic container and the contaminated pumice. Wheels are removed, rinsed under water, and bagged for autoclaving.
Before returning to the main clinic, all items arc disinfected by immersion or spray and placed in a Lock-Tight bag. All information regarding disinfection procedures performed on Prosthodontic items sent to an outside laboratory is clearly written on the prescription form and the plastic bag.
Conclusion
Dental safety is a key area of concern and needs to be addressed on top priority. The rationale for infection control is to “control” iatrogenic, nosocomial infections among patients, and potential occupational exposure of care providers to disease causing microbes during provision of care. Lack of Infection Control is life-threatening for both the patient and the Dental Professional and requires more efforts. Sterilisation & Disinfection of patient care instruments & material used are part of Infection control protocol in health care setting including dental care. Formal programs in Infection Control and Safety must be developed and strictly followed by the entire dental health care professional. Dentists must ensure that at least six basic infection control procedures should be observed when treating patients.
References
1) Textbook of Microbiology – Ananthanarayan & Paniker 6th Edition.
2) Prescott, Hartey & klein’s Microbiology – Willey, Sherwood, Woolverton, 7th Edition.
3) Principles & Practice of disinfection, preservation & sterilisation – Russel, Hugo &Ayliffe’s 5th Edition.
4) Safety standards & infection control for dental hygienists – Ellen dietz.
5) Sterilization & disinfection of dental instruments- ADA (2009).
6) Guidelines for infection control in Dental healthcare settings-2003 , MMWR Recommendations & report : Dec 19,2003.
7) Guideline for disinfection & sterilization in healthcare facilities- CDC(2008).
8) Infection control glossary – Center for Disease Control & Prevention.
9) Advancing infection control in dental care settings – Jennifer L Cleveland, JADA, 2012; 1127-1138.
10) Infection control in prosthodontics – Neeraj rampal , JOHCD, 2010; 7-11.
11) Infection control in dentistry – Porter S.R., Curr Opin Dent. 1991; 429-35.
12) Infection control in prosthodontic clinic – Naveen B.H, Journal of Dental Sciences & Research ;93-107.
13) Sterilization effect of atmospheric pressure non- thermal air plasma on dental instruments – Su Jin Sung, J Adv Prosthodont, 2013;2-8.
14) Infection control in Prosthodontics – R Narendra kumar, JIADS,2010;22-25.
15) http://www.cdc.gov/osels/scientific_edu/SS1978/Lesson1/Section10.html : Principles of Epidemiology in Public Health Practice, 3rd Edition
16) Infection control in dentistry; Dental Clinics of North America1996;40(2):114-18.
17) Robert M Brandt, James P Cofey: Infection control in a Prosthodontic residency program. J Prosthodont 1993;2:57-55
18) Council on dental materials, instruments, and equipment, council on dental practice, council on dental therapeutics. “Infection control recommendations for the dental office and the laboratory”. J. Am. Dent. Assoc., 1998; 116:241 -248.
19) Favero MS, Bond WW. Chemical disinfection of medical and surgical materials. In: Block SS, ed. Disinfection, sterilization, and preservation. Philadelphia: Lea & Febiger, 1991:617-41.
20) Dr. Anil Kohli, Dr. Raghunath Puttaiah: Infection Control & Occupational Safety Recommendations for Oral Health Professionals in India 2007
21) Cross-infection hazards associated with the use of pumice in dental laboratories. S. Witt, P. Hart ; Journal of Dentistry Volume 18, Issue 5, October 1990, Pages 281–283.
22) Westerholm HS, Bradley, Jr DV, Schwdrtz RS. Efficacy of various spray disinfcctants on irreversible hydrocolloid impressions. Int J Prosthodont 1992;5:47-54.
23) Cross-Contamination Control in Prosthodontic Practice, Clare Connor; Intj Prosthodont 1991:4:337-344.
24) British Dental Association: Guide to Blood Borne Viruses and the Control of Cross Infection in Dentistry, 1989.
25) Hiolinari JA, Mdinari GE. Is mouth rinsing before dental procedures worthwhile? J Am Dent Assoc 1992;123:75-80.
26) Stern MA, Whitacre R): Avoiding cross-contamination in prosthodontics. J Prosthet Dent 1981;46:120-122.
27) Westerholm HS, Bradley, Jr DV, Schwdrtz RS. Efficacy of various spray disinfectants on irreversible hydrocolloid impressions. Int J Prosthodont 1992;5:47-54.
28) Bergman B. Disinfection of prosthodontic impression materials: A literature review. Int J Prosthodont 1989;2:536-542.
29) Maria del Pilar Rios et al .Effects of chemical disinfectant solutions on the stability and accuracy of the dental impression complex.JProsthet Dent 1996;76:356-62.
30) Sarma AC, Neiman R. A study on the effect of disinfectant chemicals of physical properties of die stone. Quintessence Int 1998;1:53-59.
31) Naylor WP. Prosthodontic items of interest. Int J Prosthodont. 1992;5:188-89.
32) Fong. P.G., and Walter. J.D. “The effects of an immersion disinfection regime on rigid impression materials”. mt. j. Prosthodont, 1990; 3: 522 -527.
33) Jurgen setz and Peter Heeg. “ Disinfection of Pumice”. J. Prosthet. Dent., 1996; 76: 448 - 450.
34) Warfield DK, Bryington SQ: Ultrasonic potentialion of the sporicidal activity of glutaraldehyde. Oral Surg Oral Med Oral Páf/io/1982;53:342-346.
|