Introduction
In our previous article, we discussed aboutthe different types of Stem cells: viz: embryonic stem cells, adult stem cells and dental stem cells and their plasticity.As we all are well aware of the fact that oral tissues are affected by inherited disorders, trauma and neoplastic or infectious diseases. So the present trend advocates the regeneration or replacement of the affected oral tissue which is expected to solve many dental problems. Within the next 25 years unparalleled advances in dentistry are set to take place with the availability of artificial teeth, bone and oral tissues using advances in stem cell research.[1] Recent advances in dental stem cell biotechnology and cell mediated murine tooth regeneration have encouraged researches to explore the potential for regenerating living teeth with appropriate functional properties.[2]
One novel approach to restore tooth structure is based on regenerative endodontic procedures by application of tissue engineering. The key elements of tissue engineering are stem cells, morphogen (growth factor), and a scaffold of extracellular matrix.[3] Regenerative endodontic procedures can be defined as biologically based procedures designed to replace damaged structures, including dentin and root structures, as well as cells of the pulp-dentin complex. The purpose of this article is to put an insight into Stem cell markers, the potentialapplication of dental stem cells in craniofacial region and stem cell therapies in medicine to treat other systemic diseases, the recruitment strategies for use in regeneration and tissue engineering, stem cell banking and future prospects.
Markers To Identify Stem Cells:
when scientists examined stem cells under a microscope, they look just like any other cell in the tissue where they are found. So, how do scientists identify these rare type of cells found in many different cells and tissues—a process that is much akin to finding a needle in a haystack? The answer is rather simple i.e. thanks to stem cell "markers."
What are stem cell markers?
Coating the surface of every cell in the body are specialized proteins, called receptors that have the capability of selectively binding or adhering to other "signaling" molecules. There are many different types of receptors that differ in their structure and affinity for the signaling molecules. Normally, cells use these receptors and the molecules that bind to them as a way of communicating with other cells and to carry out their proper functions in the body. These same cell surface receptors are the stem cell markers.[4]
Researchers use the signaling molecules that selectively adhere to the receptors on the surface of the cell as a tool that allows them to identify stem cells. Many years ago, a technique was developed to attach to the signaling molecule to another molecule (or the tag) that has the ability to fluoresce or emit light energy when activated by an energy source such as an ultraviolet light or laser.[4] At the researchers' disposal are multiple fluorescent tags which emit light that differ in color and intensity. (Figure1).
 | Fig 1 : Identifying Cell Surface Markers Using Fluorescent Tags
 |
There are two approaches of using the combination of the chemical properties of fluorescence and unique receptor patterns on cell surfaces to identify specific populations of stem cells[4],[5]. One approach for using markers as a research tool is with a technique known as fluorescence-activated cell sorting (FACS).
A FACS instrument is used to sort out the rare stem cells from the millions of other cells. With this technique, a suspension of tagged cells (i.e., bound to the cell surface markers are fluorescent tags) is sent under pressure through a very narrow nozzle—so narrow that cells must pass through one at a time. Upon exiting the nozzle, cells then pass, one-by-one, through a light source, usually a laser, and then through an electric field. The fluorescent cells become negatively charged, while nonfluorescent cells become positively charged. The charge difference allows stem cells to be separated from other cells.[4],[5] (Figure 2).
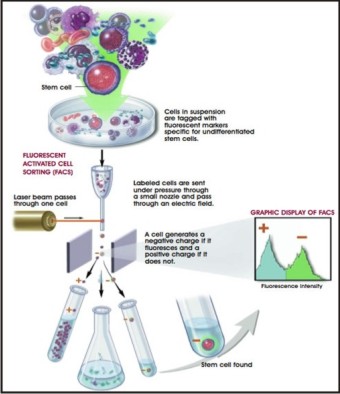 | Fig 2 : Looking For A Needle In A Haystack: How Researchers Find Stem Cells
 |
A second method uses stem cell markers and their fluorescent tags to visually assess cells as they exist in tissues. To assess how stem cells appear in tissues and in doing so a microscope is used to evaluate them rather than the FACS instrument. In this case, a thin slice of tissue is prepared, and the stem cell markers are tagged by the signaling molecule that has the fluorescent tag attached. The fluorescent tags are then activated either by special light energy or a chemical reaction. The stem cells will emit a fluorescent light that can easily be seen under the microscope.[5]
Recently, an advanced genetic engineering approach that uses fluorescence, but not dependent on cell surface markers, is being applied. The importance of this new technique is that it allows the tracking of stem cells as they differentiate or become specialized. Scientists have inserted into a stem cell a "reporter gene" called green fluorescent protein or GFP. The gene is only activated or "reports" when cells are undifferentiated and is turned off once they become specialized. Once activated, the gene directs the stem cells to produce a protein that fluoresces in a brilliant green color. Researchers are now coupling this reporting method with the FACS and microscopic methods described earlier to sort cells, identify them in tissues, and now, track them as they differentiate or become specialized.[6] (Figure 3).
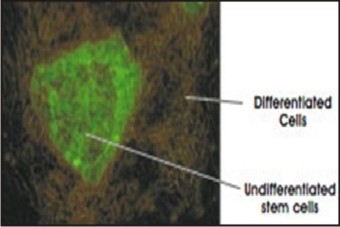 | Fig 3 : Microscopic Image Of Fluorescent-labeled Stem Cell.
 |
Limitations of stem cell markers:
One of the major limitations is a single marker identifying pluripotent stem cells, those stem cells that can make any other cell, has yet to be found.
Stem Cell Therapy: Stem cell therapies are being used quite often to treat a wide variety of diseases.Different types of stem cell therapies available are:
Bone Marrow Stem Cell Therapy: Currently the best-known and widely used therapy, bone marrow transplant is used to treat leukemia and other types of cancers as well as various blood disorders. Leukemia is a cancer of white blood cells (also called leukocytes). It occurs when leukocytes begin to grow and function abnormally, becoming cancerous. Successful treatment for leukemia depends on removal of all the abnormal leukocytes in the patient and allowing healthy ones to grow in their place. To do this, the patient's existing bone marrow and abnormal leukocytes are first killed using a combination of chemotherapy and radiation and the donor bone marrow containing healthy stem cells is introduced into the patient's bloodstream. The therapy is deemed successful when the stem cells migrate into the patient's bone marrow and begin producing new, healthy leukocytes to replace the abnormal cells.[8],[9]
Umblical Cord Blood Stem Cell Therapy: Cord blood is the blood left over in the umbilical cord and placenta after the birth of a baby. This blood has been found to be an extremely rich source of stem cells. Traditionally discarded as after-birth, umbilical cord blood is a rich source of multi-potent stem cells that have proven to be useful in treating the same types of medical conditions as those treated using bone marrow stem cells and peripheral blood stem cells. Umbilical cord blood is collected soon after birth and stored in a stem cell bank, where stem cells are harvested from the cord blood and cryo-preserved at -196oC. Umbilical cord blood stem cells hold immense potential for stem cell therapies, due to their versatile nature and easy availability. While compared to bone marrow or peripheral blood stem cells, they are less prone to rejection as they have not yet developed the features that can be recognized and attacked by the recipient's immune system. Also, as umbilical cord blood lacks well-developed immune cells, there is a lesser chance of graft versus host rejection. [10]
Peripheral Blood Stem Cell Therapy: Multi-potent peripheral blood stem cells can be used to treat leukemia & other cancers and various blood disorders. Researchers have devised a way of injecting 'growth factors' to cause these stem cells to grow faster and enter the blood. The stem cells are removed from the circulating blood through a process called apheresis. Blood is drawn from a donor's arm and is simultaneously run through a special machine that separates out the stem cells. The rest of the blood is returned to the donor and the process is repeated for a few days until enough stem cells are collected. Peripheral blood stem cells are easier to obtain than bone marrow as they can be drawn from blood. Though it is a less invasive option, but, collecting enough for therapy can be a challenge as they are found in very low quantities.[5],[11]
Hospitals that offer Stem Cell Therapy in India[12]
Adyar Cancer Hospital: Chennai ,AIIMS: New Delhi ,Armed Forces Medical College: Pune, Apollo Specialty Hospitals: Chennai, Apollo Hospitals: Hyderabad, CMC: Vellore, Global Hospitals: Hyderabad, Inlanks Hospital: Pune, Narayanan Hrudayalaya: Bangalore, NIMS: Hyderabad, R&R Army Hospital: New Delhi, Sanjay Gandhi PGIMS: Lucknow, Tata Memorial Hospital: Mumbai, TriCell SRMC: Chennai
Diseases Treated With Stem Cell Therapy:
Virtually everyone could benefit from stem cell therapies whether directly, or indirectly. Across the world, stem cell transplants have been used since the 1960s to treat a variety of diseases.[4] (Table 1).
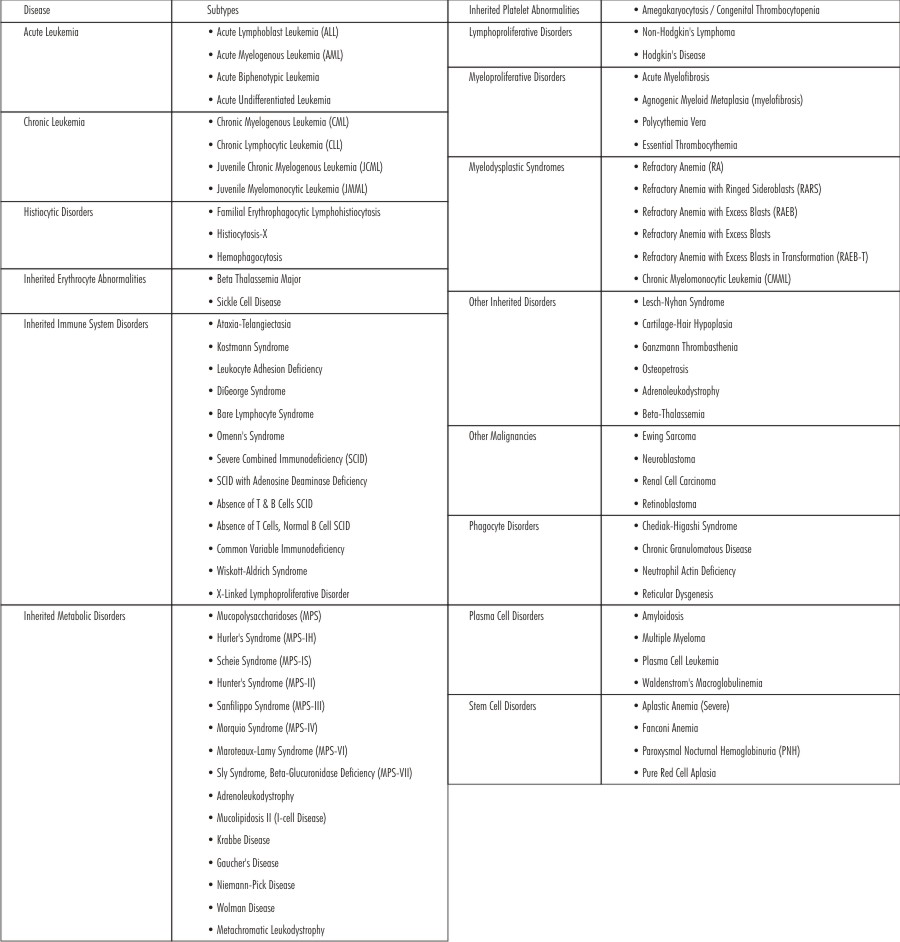 | Table 1 : Stem Cell Therapies Are Available For The Enumerated Diseases
 |
Potential Clinical Applications of Stem Cells in the Oro- Facial Complex
Craniofacial tissue engineering promises the regeneration or de novo formation of dental, oral, and craniofacial structures lost to congenital anomalies, trauma, and diseases. Several craniofacial structures such as mandibular condyle, calvarial bone, cranial suture, and subcutaneous adipose tissue have been engineered from mesenchymal stem cells. (Figure 4).
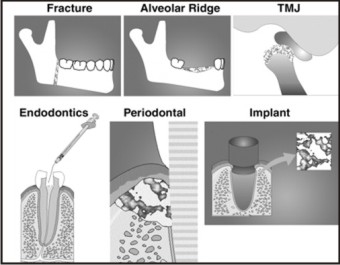 | Fig 4 : Cell-based Applications For Cranio-oro-facial Reconstruction
 |
1) In continued root formation/ Stem cell mediated root regeneration[13]
2) In pulp healing and regeneration[13]
3) In reimplanation and transplantation [13]
4) Pulp/dentin tissue engineering and regeneration[13]
5) Bioroot engineering and reconstruction of the periodotium.[13]
6) Direct orthotopic transplantation into segmental defects
7) Alveolar ridge augmentation
8) Vascularized bone grafts
9) Tissue engineering of temporo mandibular joint from the stem cells
10) Re-growing dental enamel from cultured cells
1.In continued root formation/ Stem cell mediated root regeneration
Stem cell-mediated root - regeneration offers opportunities to regenerate a bio-root and its associated periodontal tissues, which are necessary for maintaining the physiological function of teeth. A bio-root periodontal complex can be built up by postnatal stem cells including stem cells from root apical papilla (SCAP) and PDLSCs, to which an artificial porcelain crown can be affixed.[13] In a study conducted by Wataru Sonoyama et al (2006), using a minipig model, both human SCAP and periodontal ligament stem cells (PDLSs) were transplanted to generate a root/periodontal ligament complex capable of supporting a porcelain crown.[2] Further research is needed to support the role of stem cells from apical papilla in continued root formation.
2. In pulp healing and regenration
Traditional approaches to manage young permanent non vital tooth or apical periodontitis with sinus tract formation along with total pulpal necrosis recommended apexification because there is little to no expectation to continued root development. To the phenomenon of physiological root formation and regenration with the help of dental stem cells known as revascularization by Iwaya et al and Banchs & Trope. [14]
3. In reimplanation and transplantation
Stems cells helps in reimplantation and transplantation, this fact is supported by Skoglund et al, Andreasen et al and king et al. Skoglund et al observed revascularization of the pulp of replanted and autotransplanted teeth with incomplete root development in dogs. Ingrowth of new vessels occurs during the first few postoperative days. After 10 days, new vessels are formed in the apical half of pulp and after 30 days in the whole pulp.[15] Andreasen et al and king et al showed excellent radiographic images of the ingrowth of bone and periodontal ligament next to the inner dentinal wall into the canal space with arrested root formation after the replantation of avulsed maxillary incisors, suggesting a complete loss of the viability of pulp, apical papilla and/of HERS.[16]
4. Pulp/dentin tissue engineering and regeneration
Dental pulp tissue engineering was first tested by Mooney’s groups. Bohl et al reported that culturing pulp cells grown in vitro on poly glycolic acid (PGA) resulted in high cell density tissue similar to the native pulp. Burma et al found that pulp cells seeded in PGA and implanted into the subcutaneous space of immune compromised mice produced extracellular matrix. New blood vessels also penetrated the cells/PGA implants in vivo, 3 weeks after the implantation.[17] Since then the isolation and characterization of DPSCs and SHED using these stem cells for dentin/ pulp tissue regeneration has drawn great interest. These finding provide new light on the possibility of generation pulp and dentin in pulpless canals.
5. Regenerating human periodontal ligament
Seo et al[18] discovered stem cells from human periodontal ligament. These stem cells have the potential to generate periodontal ligament and cementum. In another research, mesenchymal stem cells have been used to treat periodontal defects (Hasegawa et al).[19] They transplanted bone marrow-derived mesenchymal stem cell to experimental ClassIII periodontal defects. Four weeks after transplantation, the periodontal defects were almost regenerated with periodontal tissue. In the regenerated periodontal tissues cementoblasts, osteoblasts, osteocytes, and fibroblasts were detected.
6. Direct orthotopic transplantation into segmental defects
Investigators have developed a number of animal models of segmentaldefects in mice, dogs, and sheep. In sheep, ceramic blocksloaded with BMSCs were found to completely heal long-bone defects.[21] In dogs, investigators created critical-size defectsin the cranium and filled them with ex vivo expanded BMSCs eitherin collagen sponges or in association with HA/TCP .In both cases, the defects healed completely, and the newlyformed bone integrated into the margin.[22]
7. Alveolar ridge augmentation
Restoration of alveolar ridge height is of utmost concern topracticing dentists in trying to prevent the loss of a toothdue to bone destruction induced by periodontal disease, andin maintaining the ability of edentulous patients to wear dentures. Appropriate ridge height is also is essential for the placementand long-term retention of dental implants. Standard practiceinvolves the use of autologous or allogenic bone grafts, orceramics, both with and without growth factors, but the outcomesare variable. In animal models, BMSCs are used in conjunction withHA/TCP. They have been successful in building alveolar bone. With further refinement, these types of procedureswould mark a major advancement in dental reconstruction.[23] (Figure 5).
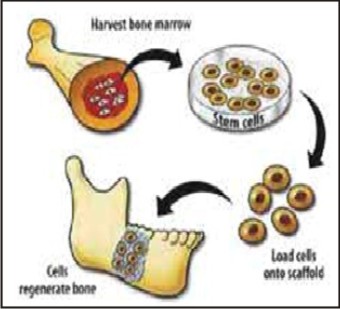 | Fig 5 : Stem Cells Harvested From Bone Marrow To Regenerate Bone
 |
8. Vascularized bone grafts
Researchers[24] have developed methods to generatevascularized bone grafts by placing BMSCs in collagen sponges,which then are wrapped around an artery and vein. The collagensponge containing cells subsequently is wrapped with Teflon(Dupont Teflon, Wilmington, Del.) to prevent blood vessel ingrowthfrom the side (collateral ingrowth). After several weeks, thesebone rudiments are found to be perfused entirely by the arteryand vein that they surround, and they then can be moved to anothersite where the blood vessels can be reattached to existing bloodvessels in the margins of the recipient site.
9. Tissue engineering of temporomandibular joint from the stem cells
In the past few years, we have reported the tissue engineeringof a mandibular condyle exhibiting the shape and dimensionsof a human cadaver TMJ. MSCs were isolated from femoral and tibial bone marrows of adultrats and exposed separately to either chondrogenic or osteogenic supplemented culture medium (Alhadlaq and Mao, 2003; Alhadlaq et al., 2004).[25],[26] De novo formation of a structure having the same shape and dimensionsas the cadaver human mandibular condyle was observed after 4wks of in vivo implantation. The tissue-engineeredmandibular joint condyles retained the macroscopic shape anddimensions of the cadaver mandibular condyle .
10. Re-growing dental enamel from cultured cells
Dental enamel cannot regenerate itself, because it is formed by a layer of cells that is lost by the time the tooth appears in the mouth. In the study being reported today, the researchers seeded the cultured dental epithelial cells onto collagen sponge scaffolds, along with cells from the middle of the tooth (dental mesenchymal cells). The scaffolds were then transferred into the abdominal cavities of rats, where conditions were favorable for the cells in the scaffolds to interact and develop. When removed after 4 weeks, the remnants of the scaffolds were found to contain enamel-like tissue. The key finding of this study was that even after the multiple divisions that occurred during propagation of the cells in culture, the dental epithelial cells retained the ability to produce enamel, as long as they were later provided with an appropriate environment.[27]
Tissue Engineering: Langer and Vacanti[28] defined tissue engineering as an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function. The key elements of tissue engineering are stem cells, Growth factor (morphogen) and a scaffold of extracellular matrix. (Figure 6).
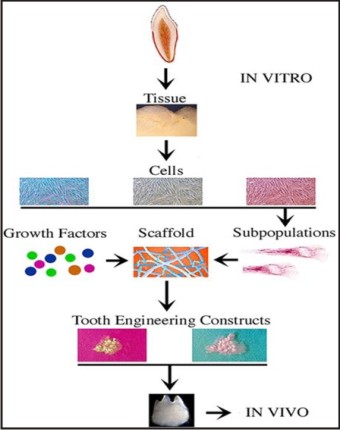 | Fig 6 : Construction Of A Bioengineered Tooth.
 |
a) Stem cells: A population of high quality human stem cells was found in the exfoliated human primary teeth, recently (Miura et al, 2003).[29] SHED demonstrateda strong capacity to induce recipient cell-mediated bone formationin vivo. SHED could not differentiate directly into osteoblasts but did induce new bone formation byforming an osteoinductive template to recruit murine host osteogeniccells. These data imply that deciduous teeth may not only provideguidance for the eruption of permanent teeth, as generally assumed,but may also be involved in inducing bone formation during theeruption of permanentteeth.[29] Previous experiments haveshown that dental pulp tissue of adult teeth contains a populationof DPSCs that are capable of differentiating into odontoblastsand adipocytes as well as expressing nestin and glial fibrillary acidic protein (GFAP) and forma dentin/pulp-like complex after in vivo transplantation.[30] Deciduous teeth are significantly different from permanent teethwith regards to their developmental processes, tissue structure,and function.[29] Therefore, it is not a surprise to find that SHEDare distinct from DPSCs with respect to their higher proliferationrate, increased cell-population doublings, sphere-like cell-clusterformation, osteoinductive capacity in vivo, but failure to reconstitutea dentin-pulp-like complex , perhaps in order to have more immature characteristics than other post-natal stem cell population .[29]
b) Scaffolds: The second component of tissue engineering is a physical scaffold. Tissues are three-dimensional structures, and an appropriate scaffold is needed to promote cell growth and differentiation. It is known that extracellular matrix molecules control the differentiation of stem cells, and an appropriate scaffold might selectively bind and localize cells, contain growth factors, and undergo biodegradation over time.[31]
Scaffold requirements:To achieve the goal of pulp tissue reconstruction, scaffolds must meet some specific requirements: (a) A scaffold should contain growth factors to aid stem cell proliferation and differentiation, leading to improved and faster tissue development (b) Scaffold should be effective for transport of nutrients, oxygen, and waste. It should be gradually degraded and replaced by regenerative tissue, retaining the feature of the final tissue structure (c) A high porosity and an adequate pore size are necessary to facilitate cell seeding and diffusion throughout the whole structure of both cells and nutrients.[32]
Scaffold Materials
Ø Platelet-rich plasma (PRP)
Ø Bone sialoprotein.[33]
Ø Alginate hydrogel
Ø Mineral trioxide aggregate (MTA).[34]
Ø The synthetic materials include polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL).[35]
Ø Synthetichydroxyapatite/tricalcium phosphate (HA/TCP) ceramics
c) Growth factors: The third components of tissue engineering are morphogen. Morphogen can be used to control stem cell activity, such as by increasing the rate of proliferation, inducing differentiation of the cells into another tissue type, or stimulating stem cells to synthesize and secrete mineralized matrix. A variety of growth factors have successfully been used for dentin-pulp complex regeneration, including transforming growth factors –beta (TGFs),[36] bone morphogenetic proteins (BMPs),[37] platelet-derived growth factor (PDGF),[38] insulin-like growth factor (IGF),[39] fibroblast growth factor,[3] vascularendothelial growth factor[3] and prostaglandin E2[3],[36] - all havebeen demonstrated to influence bone formation and vascular ingrowthinto various scaffoldings.
Summary of Tooth Injury and Possible Applications of Tissue-engineering Approaches to Aid Healing. (Table 2).
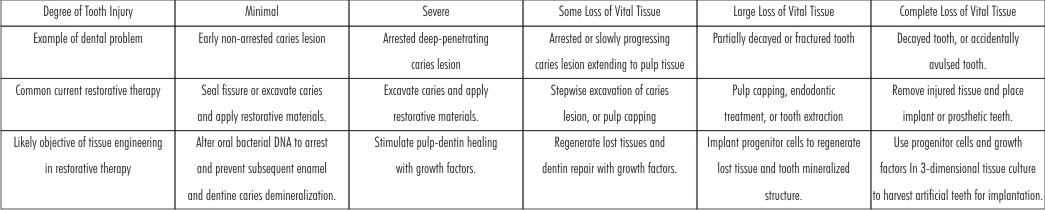 | Table 2 : Summary Of Tooth Injury And Possible Applications Of Tissue-engineering Approaches To Aid Healing
 |
Stem Cell Banking
Licensed tooth stem cell banks, Internationally and in India, used for cryopreservation and isolation are as follows[12]
1. In Japan, the first tooth bank was established in Hiroshima University and the company was named as ‘ThreeBrackets’ (Suri Buraketto).
2. BioEden (Austin, Texas), StemSave, and StoreR09;aR09;Tooth (USA)
3. The Norwegian tooth bank.
4. In India, Stemade Biotech Pvt. Ltd. (Delhi, Chennai, Chandigarh, Pune, and Hyderabad). In India, ‘Life Cell International’, was the first private sector stem cell banking services started in Bangalore (India) in the year 2009.
Challenges
Stem cell research has undergone huge advancements in the past couple of years. This does not mean, however, that researchers have not faced problems. Following are few major hurdle while using stem cells:[40]
1) Its challenging for scientists to ensure the long term proliferative ability and pluripotency of embryonic stem and germ cells.
2) It is necessary to understand the unique genetic and molecular basis by which these cells are able to replicate indefinitely.
3) Culturing stem cells requires sufficient quantities of stem cells to treat specific diseases.
4) Teratoma formation has also produced a hurdle that needs to be overcome.
5) Immune challenges also prove a significant barrier to the application of stem cell therapies. If the stem cells are recognized as non-self, they will be rejected and destroyed.
6) Adult stem cells have also presented unique problems of their own to the researchers. Not only they are difficult to maintain in culture, like the embryonic stem cells, but also are very rare in adult tissues .Thus adult stem cells are very difficult to isolate and identify.
Prospects
Clearly, advances in adult stem cell biology have provided a great deal of impetus for the biomedical community to translate these findings into clinical application. Given the fact that we have in hand populations of stem cells that reproducibly reform bone and its marrow, cementum, dentin, and perhaps even periodontal ligament, it is possible to envision complete restoration of the hard tissues in the oral cavity using the patient’s own cells, thereby avoiding issues of histocompatability. Furthermore, advances in techniques to genetically modify the gene activity of stem cells during their ex vivo expansion offers the unique possibility to make a patient’s own stem cells even better. However, replacing dental tissues with either cell- or gene-based therapy may be complicated in areas of unresolved inflammation, thus highlighting the need for more research to understand potential complicating factors.
Conclusion
The recent recognition of stem cells and their role in tissue regeneration provide a strong basis upon which we can begin to actually depend on for the clinical management of craniofacial defects. The technical hurdles to achieve above mentioned goals should not be underestimated. The kind of scaffold, the sourceof cells, the type of in vitro culturing, type of surgical procedureto be used-all require careful consideration. Kabir, et al.[41] wrote “If bone marrow is the site of first choice for hematopoietic stem cell collection, dental pulp must be considered one of the major sites for mesenchymal cell collection”. So here I conclude stem cells may possibly have a key role in not only management of many degenerative diseases but also in regenerative endodontics.
References
1. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31: 711-18.
2. Wataru Sonoyama.Mesenchymal stem cell mediated functional tooth regeneration in swine. Journal Pone. Dec 20, 2006.
3. Malhotra N, Mala K.Regenerative endodontics as a tissue engineering approach: Past, current and future. Aust Endod J 2012;38:137R09;48.
4. Bianco P, Robey PG.Skeletal stem cells. In: Lanza RP, ed. Handbook of adult and fetal stem cells. San Diego: Academic Press; 2004: 415–24
5. "Appendix E: Stem Cell Markers". Stem Cell Information. Bethesda, Maryland: National Institutes of Health. 17 June 2001. Retrieved 23 July 2009
6. Koo, J.; Kim, Y.; Kim, J.; Yeom, M.; Lee, I. C.; Nam, H. G.(2007). "A GUS/Luciferase Fusion Reporter for Plant Gene Trapping and for Assay of Promoter Activity with Luciferin-Dependent Control of the Reporter Protein Stability". Plant and Cell Physiology.2007; 48 (8): 1121–1131.
7. De Bari C, Dell’accio F. Cell therapy: A challenge in modern medicine. Biomed Mater Eng 2008; 18 : 11-17.
8. Holtzer, H.(1978). Cell lineages, stem cells and the ‘quantal' cell cycle concept. In: Stem cells and tissue homeostasis. Eds: B.I. Lord, C.S. Potten, and R.J. Cole. (Cambridge, New York: Cambridge University Press). 1–28
9. Pavletic SZ, Khouri IF, Haagenson M, et al."Unrelated donor marrow transplantation for B-cell chronic lymphocytic leukemia after using myeloablative conditioning: results from the Center for International Blood and Marrow Transplant research". J. Clin. Oncol.2005; 23 (24): 5788–94.
10. Kang KS, Kim SW, Oh YH, et al."A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study". Cytotherapy. 2005; 7 (4): 368–73.
11. Schachna L, Ryan P F, Schwarer A P.MalignancyR08;associated remission of systemic lupus erythematosus maintained by autologous peripheral blood stem cell transplantation. Arthritis Rheum. 1998.412271-2272.2272.
12. Arora V, Arora P, Munshi AK.Banking stem cells from human exfoliated deciduous teeth (SHED): Saving for the future. J Clin Pediatr Dent 2009;33:289R09;94….Hospital.
13. Krishna V, Madathanapalli S, Vaishali S, Rahul S.Stem cells-The future of dentistry: A review. JIAOMR, July-Sept 2011; 23(3): S370-372.
14. Banchs F and Trope M.Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? 2004;30(4):196-200.
15. Skoglund A, Tronstad L.Pulpal changes in replanted and autotrans-planted immature teeth of dogs. J Endodon 1981;7: 309-316.
16. Peter E Murra, Franklin Garcia –Godoy, Kenneth M Hargreaves.Regenerative endodontics: A review of current status and a cal for action. JOE.2007;33(4).
17. Brett Peterson, Jeffery Zhang et al.Healing of the critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue engineering. 2005; 11:120-129.
18. Seo BM, Miura M, Gronthos S et al.Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004; 364(9429): 149–55.
19. Hasegawa M, Yamato M, Kikuchi A, Okano T et al.Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng.2005; 11: 469–478.
20. Kon E, Muraglia A, Corsi A, et al.Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res 2000; 49(3): 328–37.
21. Krebsbach PH, Kuznetsov SA, Satomura Ket al. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 1997; 63(8): 1059–69.
22. Treena Livingston Arinzeh, Susan J. Peter, Michael P. Archambault, Christian van den Bos, Steve Gordon, Karl Kraus, Alan Smith, Sudha Kadiyala.Allogeneic Mesenchymal Stem Cells Regenerate Bone in a Critical-Sized Canine Segmental Defect. J Bone Joint Surg Am, 2003 Oct;85(10):1927-1935.
23. Ueda M, Yamada Y, Ozawa R, Okazaki Y.Clinical case reports of injectable tissue-engineered bone for alveolar augmentation with simultaneous implant placement. Int J Periodontics Restorative Dent 2005; 25(2):129–37.
24. Warnke PH, Springer IN, Wiltfang J, et al.Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004; 364(9436): 766–70.
25. Alhadlaq A, Mao JJ.Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004; 13: 436–448.
26. Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003; 82: 951–956.
27. Science Daily:Seintists re-grow dental enamel from cultured cells. 2007. Source: Internet: www.sciencedaily.com.
28. Langer R, Vacanti JP.Tissue engineering. Science. 1993 May;260(5110):920-6.
29. Miura M, Gronthos S, Zhao M, Lu Bet al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 2003; 100(10): 5807–12.
30. Sonoyama W, Liu Y, Fang D et al.Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006; 1: e79.
31. Graziano A, d’Aquino R, Cusella-De Angelis MG, de Francesco F, Giordano A, Laino G et al.Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J Cell Physiol. 2008 Jan;214(1):166-72.
32. Karande TS, Ong JL, Agrawal CM. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann Biomed Eng. 2004 Dec;32(12):1728-43.
33. Decup F, Six N, Palmier B, Buch D, Lasfargues JJ, Salih E et al. Bone sialoprotein-induced reparative dentinogenesis in the pulp of rat’s molar. Clin Oral Investig. 2000 Jun;4(2):110-9.
34. Torabinejad M, Chivian N.Clinical applications of mineral trioxide aggregate. J Endod. 1999 Mar;25(3):197-205.
35. Athanasiou KA, Niederauer GG, Agrawal CM.Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996 Jan;17(2):93-102.
36. Dobie K, Smith G, Sloan AJ, Smith A.Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect Tissue Res. 2002;43(2-3):387-90.
37. Sloan AJ, Rutherford RJ, Smith AJ.Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000 Feb;45(2):173-7.
38. Yokose S, Kadokura H, Tajima N, Hasegawa A, Sakagami H, Fujieda K et al. Platelet-derived growth factor exerts disparate effects on odontoblast differentiation depending on the dimers in rat dental pulp cells. Cell Tissue Res. 2004 Mar;315(3):375-84.
39. Lovschall H, Fejerskov O, Flyvbjerg A.Pulp-capping with recombinant human insulin- like growth factor I (rhIGF-I) in rat molars. Adv Dent Res. 2001 Aug;15:108-12.
40. G Amit, G Taru, Madan N. Dental pulp stem cells in endodontics research: a promising tool for tooth tissue engineering, RSBO. 2011:8(3): 335-337.
41. Ramchandra Kabir, Manish Gupta, Avanti Aggarwal, Deepak Sharma, Anurag Sarin, Mohammed Zaheer Kola.Imperative Role of Dental Pulp Stem Cells in Regenerative Therapies: A Systematic Review. 2014; 20(1).
|