Introduction
The harmful effect of microorganisms present in pulp and periapical pathologies has been studied since 1894, when Miller[1] proved the presence of bacteria in the interior of root canals and observed their importance in the etiology of such alterations. This was then confirmed by posterior studies [2], [3].
The use of chemical irrigants during chemo-mechanical canal preparation is important for disinfection and cleaning of the canal system[4]. As access to the root canal system is limited and the anatomy complex, microorganisms may remain in the dentinal tubules and in other irregular spaces. When these microorganisms find a supporting environment, they can proliferate and re-infect the root canal system. Studies have demonstrated that chemo-mechanical preparation combined with a non antiseptic irrigating solution was capable of reducing approximately 50% of bacteria in root canals[5]. While sodium hypochlorite (NaOCl) solution eliminated approximately 80% of bacteria.
An irrigant serves to flush out debris from within the instrumented root canals, dissolve organic tissue remnants, disinfect the root canal space and provide lubrication during instrumentation, without causing irritation to biological tissues[6],[7]. Sodium hypochlorite (NaOCl) has become the most popular agent for endodontic irrigation, even though its optimum working concentration has not been universally agreed[6]. Chlorhexidine gluconate (CHX), a less malodorous and toxic agent, has been suggested as an irrigant based on its antibacterial effects, substantivity and lower cytotoxicity than NaOCl, whilst demonstrating efficient clinical performance[8].
One of the most commonly used antimicrobial activity tests is the agar diffusion test, which involves the placement of paper disks, previously saturated with solutions, on Petri dishes containing agar with a selected microorganism. After a certain period of time, zones of inhibition of variable diameters form around the paper disks if the tested substance presents antimicrobial activity[9].
The purpose of this study was to evaluate, in vitro, the antimicrobial activity of NaOCl (1% and 3%) and CHX (1% and 2%) solutions against strains of bacteria found in infected root canals including facultative anaerobic microorganisms (E. Faecalis and S. aureus) using agar diffusion test and also to determine the influence of an organic load material. Sterile plasma (organic material) was added to simulate organic tissue present in the root canal system.
Material And Method
Sodium hypochlorite with initial concentration of 4% was diluted in sterile water to make final concentration of 1% and 3% and Chlorhexidine gluconate with initial concentration of 20% was diluted in sterile water to make final concentration of 1% and 2% respectively. Sterile plasma (0.5%) was added as organic load material.
Plates containing Muller–Hinton agar were seeded with bacterial strains Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212). Paper disks (7-mm diameter) saturated with 10 μL of test solution for 1 min were placed on the plates. In each Petri dish, one paper disk soaked with sterile saline solution was placed as negative control. Agar plates inoculated with bacterial strains were incubated for 24 hrs at 37S04;C.Diameters of the zones of bacterial inhibition were measured with a transparent ruler and recorded for each strain and solution.Test was repeated 5 times. Data were statistically analyzed using SPSS software; student’s t-test was used to compare the diameters of the zones of bacterial inhibition to determine the influence of sterile plasma on the effectiveness of the solutions (P < 0.01).Sterile plasma interfered with the antimicrobial activity of 1% CHX and 1% and 3% NaOCl against E. faecalis;and 1% and 3% NaOCl against S. aureus.
To compare the antimicrobial activity of tested solutions the ANOVA and Bonferroni’s tests (P < 0.05) were performed. In the presence of sterile plasma, 2% CHX had the most reliable antimicrobial result for all strains tested. However, in the absence of sterile plasma, 2% CHX was the most effective antimicrobial against E. faecalis and S aureus. The level of significance was kept at 0.05%.
Results
The outcome of the present study the mean diameters of the zones of bacterial inhibition for each irrigant solution against the bacterial strains, with or without the organic load, are shown in Table 1.1. All concentrations of CHX and NaOCl without organic load produced zones of antimicrobial activity against the tested strains. However, when higher concentrations of the solution were used, larger inhibition halos were formed.
Whereas samples containing organic load exhibited inhibition zones although the diameters were smaller than in the absence of organic load. Organic load interfered with the antimicrobial activity of 1%, 2% CHX and 3% NaOCl against the tested strains resulting in smaller inhibition halos. In the presence of organic load, 2% CHX had the most reliable antimicrobial result for all strains tested as shown in the bar graph Fig 1.2.
 | Table: 1.1: Arithmetical means of the diameters (mm) of the bacterial inhibition zones for Sodium Hypochlorite and Chlorhexidine solutions on microorganisms in the agar diffusion test.
 |
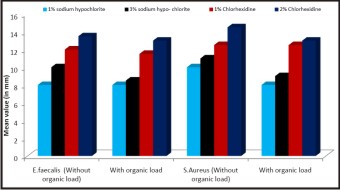 | Fig: 1.2: Bar graph representing the arithmetical means of the diameters (mm) of the bacterial inhibition zones for Sodium Hypochlorite and Chlorhexidine solutions at different concentrations on E. faecalis and Staph. aureus.
 |
Discussion
Complete debridement and disinfection of the pulpal space are considered to be essential for predictable long-term success in endodontic treatment. Residual pulpal tissue, bacteria, and dentine debris may persist in the irregularities of root canal systems, even after meticulous mechanical preparation[10]. Therefore, several irrigant solutions have been recommended for use in combination with canal preparation. However, the efficacy of these procedures also depends upon the vulnerability of the involved species. Anaerobic bacteria, especially black-pigmented Gram-negatives, have been linked to the signs and symptoms of endodontic disease[11] but facultative bacteria, such as Enterococcus faecalis, have also been isolated from pathologically involved root canals, being considered one of the most resistant species in the oral cavity and a possible cause of failure of root canal treatment[12].
Sodium hypochlorite solution is, to date, the most commonly employed root canal irrigant. However, no general agreement exists regarding its optimal concentration, which ranges from 0.5% to 5.25%. NaOCl provides good tissue solvent action[13],[14], has a broad spectrum of antimicrobial activity [4],[5],[15], acts as a lubricant for instrumentation and can flush loose debris from root canals[10]. The major disadvantages of NaOCl are its cytotoxic effect if injected into the periapical tissues[15], its foul smell and taste, its ability to bleach clothes, and it’s potential for causing corrosion[16]. It is also known to produce allergic reactions[17].
Chlorhexidine gluconate is a cationic bisguanide that seems to act by adsorbing onto the cell wall of the microorganism and causing leakage of intracellular components. At low chlorhexidine concentrations, small molecular weight substances will leak out, specifically potassium and phosphorous, resulting in a bacteriostatic effect. At higher concentrations, chlorhexidine has a bactericidal effect due to precipitation and/or coagulation of the cytoplasm, probably caused by protein cross-linking[18].
Present study evaluated the antimicrobial activity of sodium hypochlorite and chlorhexidine gluconate irrigant solutions against different bacterial strains using agar diffusion test. Sterile plasma was added as an organic load to simulate the organic content within the root canal and to verify the effectiveness of the antimicrobial activity[19]. Organic materials have been shown to interfere with the antimicrobial activity of canal irrigating solutions[20], and no previous study has analyzed the effectiveness of irrigating solutions in the presence of an organic load.
In the present study, we used strains that best represented endodontic infections and that were good models to be tested for antimicrobial sensitivity. Two facultative anaerobic bacteria strains were tested (E. faecalis and S. aureus) because these are present in all phases of the development of an infection in root canals[21],[22]. Although sodium hypochlorite is the most clinically used irrigating solution, there is no agreement about its optimum concentration[5].
Various studies tested many irrigating solutions in both in vitro and in vivo, and concluded that an ideal solution is that which combines both maximum antimicrobial effects with minimum toxicity[15].
Chlorhexidine solution presents a wide spectrum antimicrobial action, substantivity and is relatively nontoxic. However, it does not dissolve organic material.
The present study showed all concentrations of CHX and NaOCl without organic load produced zones of antimicrobial activity against the tested strains. However, when higher concentrations of the solution were used, larger inhibition halos were formed. Whereas samples containing organic load exhibited inhibition zones although the diameters were smaller than in the absence of organic load. Similar results have been reported in previous investigations[4],[23],[24]. Using the same test, Yesilsoy et al. demonstrated the antimicrobial activity of 0.12% CHX against a number of bacterial strains, including P. gingivalis. Siqueira Jr et al. reported that 0.2% and 2% CHX was antimicrobial against P. gingivalis and P. endodontalis.
Organic load interfered with the antimicrobial activity of 1%, 2% CHX and 3% NaOCl against the tested strains resulting in smaller inhibition halos. In the presence of organic load, 2% CHX had the most reliable antimicrobial result for all strains tested. A factor that might explain this finding is the possible formation of a high molecular weight substance, as a result of a reaction between the NaOCl and organic load that could hamper the diffusion of the irrigating solution through the media resulting in low or absence of inhibition zones[9]. By contrast CHX has good diffusibility through the agar gel[4],[8], [24].
Conclusion
Within the limitations of this in vitro study, it may be concluded that Chlorhexidine should be used in conjunction with sodium hypochlorite as it does not have tissue dissolving capabilities. However Chlorhexidine should be the last irrigant to be used as it has better antimicrobial efficacy as well as substantivity
Also presence of organic load does not have a major impact on its antimicrobial efficacy as compared to sodium hypochlorite.
References
1. Miller WD. An introduction to the study of the bacterio-pathology of the dental pulp. Dental Cosmos 1894; 36:505-528.
2. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. 1965; 20:340-349.
3. Sundqvist G. Bacteriological studies of necrotic dental pulps. Dissertation. University of Umea, Umea, Sweden, 1976. apud: Morse DR. Endodontic microbiology in the 1970s. Int Endod J.1981; 14:69-79.
4. Siqueira J Jr, Batista M, Fraga R, Uzeda M. Antibacterial effects of endodontic irrigants on black–pigmented gram negative anaerobes and facultative bacteria. J Endod 1998; 24: 414–16.
5. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol 1983; 55: 307–12.
6. Cheung GSP, Stock CJR (1993) In vitro cleaning ability of root canal irrigants with and without endosonics. International Endodontic Journal 26, 334–43.
7. Ingle JI, Bakland LK, Peters DL, Buchanan S, Mullaney TP (1994) Endodontic cavity preparation. In: Ingle JI, Bakland LK, eds. Endodontics . 4th edn. Baltimore, USA: Williams &Wilkins, 92–227.
8. Leonardo MR, Tanomaru Filho M, Silva LAB, Nelson Filho P, Bonifácio KC, Ito IY (1999) In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. Journal of Endodontics 25 , 167–71.
9. Tobias RS. Antibacterial properties of dental restorative materials: a review. Int Endod J 1988; 21: 155–60.
10. Abou-Rass M, Piccinino MV (1982) The effectiveness of four clinical irrigation methods on the removal of root canal debris. Oral Surgery, Oral Medicine and Oral Pathology 54, 323–8.
11. Gomes BPFA, Lilley JD, Drucker DB (1996a) Clinical significance of dental root canal microflora. Journal of Dentistry 27 , 47–55.
12. Gomes BPFA, Lilley JD, Drucker DB (1996b) Variations in the susceptibilities of components of the endodontic microflora to biomechanical procedures. International Endodontic Journal 29, 235–41.
13. Grossman L, Meiman B (1941) Solution of pulp tissue by chemical agents. Journal of the American Dental Association 28 , 233–5.
14. Senia ES, Marraro RV, Mitchell JL, Lewis AG, Thomas L (1975) Rapid sterilization of gutta-percha with 5.25% sodium hypochlorite. Journal of Endodontics 1 , 136–40.
15. Spångberg L, Engström B, Langeland K (1973) Biologic effects of dental materials. 3. Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surgery, Oral Medicine and Oral Pathology 36, 856–70.
16. Neal RG, Craig RG, Powers JM (1983) Effect of sterilization and irrigants on the cutting abilities of stainless steel files. Journal of Endodontics 9 , 93–6.
17. Kaufman AY, Keila S (1989) Hypersensitivity to sodium hypochlorite. Journal of Endodontics 15 , 224–6.
18. Fardal O, Turnbull RS (1986) A review of the literature on use of chlorhexidine in dentistry. Journal of the American Dental Association 112 , 863–9.
19. Cremieux A, Fleurette J. Methods of testing disinfectants. In: Block SS, ed. Disinfection, sterilization and preservation. 4th ed. Philadelphia, PA: Lea & Febiger Publishers; 1991. pp. 1009–27.
20. Briseño BM, Wirth R, Hamm G, Standhartinger W. Efficacy of different irrigation methods and concentrations of root canal irrigation solutions on bacteria in the root canal. Endod Dent Traumatol 1992; 8: 6–11.
21. Fabricius L, Dahlén G, Öhman AF, Möller AJR. Predominant indigenous oral bacteria isolated from infected root canals after varied times of closure. Scan J Dent Res 1982; 90:134-144.
22. Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol 1992; 7:257-262.
23. Siqueira JF Jr, Lopes HP, Magalhães FAC, Uzeda M. Efeito antimicrobiano do hipoclorito de sódio a 1% e a 5.25% sobre bacilos anaeróbios produtores de pigmento negro. Rev Paulista Odontol 1999; 1: 4–6.
24. Yesilsoy C, Whitaker E, Cleveland D, Phillips E, Trope M. Antimicrobial and toxic effects of established and potential root canal irrigants. J Endod 1995; 21: 513–15.
|