Introduction
Short Root Anomaly (SRA) is a rare developmental anomaly in the permanent dentition apparently unrelated to any systemic condition or syndrome. Its etiology is not known; however, familial occurrence has been suggested (Lind, 1972; Edwards and Roberts, 1990)[1]. The prevalence of SRA among populations range from 0.6% to 10% (Ando et al., 1967; Jakobsson and Lind, 1973; Bergström, 1977; Brook and Holt, 1978).[2],[3], [4], [5]
Hypoplasia of teeth root is an inherited disease characterized by dental short-root anomaly (SRA), which is a disorder due to the abnormal physiological development of teeth roots. It is one of the most serious causes of teeth shedding, and the incidence rate of SRA is about 1.3%[6].The prevalence is probably attributable to some genetic factor[7], Stevens-Johnson syndrome, congenital renal diseases, antineoplastic therapy, chronic dental infection, traumatic factor and orthodontic treatment. Some inherited diseases could cause HTR phenotypes. The earliest case reported was a 19-year-old boy with underdeveloped dental roots and early exfoliation of the teeth[8].
Review Of Literature
SRA was first documented by Lind (1972)[1], who described this till-then-unclassified developmental dental anomaly with abnormally short roots as always affecting both maxillary central incisors almost symmetrically. Root formation appears complete, with closed apices in the erupted teeth.
Characteristic of SRA is its strong bilateral occurrence. In the SRA cases reported, the maxillary central incisors seem always to be severely affected, usually with roots of about equal length or shorter than the crowns (Lind, 1972). Other teeth are less often involved, usually with premolars and canines affected (Ando et al., 1967; Lind, 1972)[2],[1]. Newman (1975) found that “idiopathic” root shortness usually affects maxillary incisors, maxillary premolars, and mandibular second premolars, in that order, with maxillary teeth significantly more often affected than mandibular teeth. Apart from root length, the teeth and their surrounding tissues are radiographically normal.[9]
In most cases, SRA does not present any symptoms, except that in severe cases the central incisors may have increased mobility. An increased tendency towards root resorption during orthodontic treatment and due to pressure from embedded canines has been reported in SRA dentitions (Lind, 1972; Newman, 1975). [1],[9]
There have appeared occasional reports of an association with other dental anomalies such as developmentally missing teeth (hypodontia), peg-shaped lateral incisors, invaginations, generalized microdontia, and supernumerary teeth (Lerman and Gold, 1977; Brook and Holt, 1978; Edwards and Roberts, 1990), but no reports of SRA in the primary dentition.[10],[11],[12]
Desai RS et al in 2006 reported an unusual case of generalized short roots associated with microdontia, taurodontism of posterior teeth, and multiple dens invaginatus along with short stature in a 20-year-old man, who had lost several teeth because of spontaneous exfoliation.[13]
Rajić Šikanjić in 2006 reported short root anomaly of maxillary central incisors in young female skeleton from the medieval Istria. In affected teeth, the crown: root ratio was 1:1.6.This appears to be the first documented case of anomaly from the medieval period.[14]
Prevalence of SRA seems to vary with ethnic background. Populations studied differ in size and age distribution, and the diagnostic criteria for short-rooted teeth vary. In one study of Japanese schoolchildren mean age 9 years, 10 months, the proportion with short-rooted central incisors was 10% (Ando et al., 1967)[2]. In Europe, prevalence of 2.4% and 2.7% for schoolchildren aged 11 and over has been reported, with a male: female ratio of 1:2.6 and 1:1.5 (Jakobsson and Lind, 1973; Brook and Holt, 1978). [11]
Bergström (1977) studied a sample of 2 589 school children aged 8 to 9, and found a prevalence of 0.6% for short-rooted maxillary central incisors. Girls were affected three times as often as were boys.[4]
Saini TS in 2004 reported three case reports of aberrant root formation and a review of root genesis.[15]
Genetic Factors
Isolated cases of unknown origin have appeared (Leonard, 1972; Lerman and Gold, 1977), but pedigree data from both completely and incompletely ascertained families suggest a strong familial background (Lind, 1972; Newman, 1975; Edwards and Roberts, 1990)[9],[10]. Furthermore, racial variation occurs; this racial variation, together with a strong familial occurrence, indicates that SRA has a genetic background, with an autosomal dominant pattern of inheritance (Lind, 1972; Newman, 1975; Edwards and Roberts, 1990)[10]. Because of restricted family material, no definite conclusions have been established as to the mode of inheritance.
Environmental Factors
In principle, many environmental factors such as trauma, periapical infection, or Surgical procedures may cause arrest in root development or root shortening (resorption). Dental Trauma is possibly the most important reason behind the shortening of the root of a single incisor. Among children aged 1 to 16 years, 35% experience injury to their primary or permanent dentition (Borssen and Holm, 1997)[16]. In the permanent dentition, 75% to 88% of the traumatized teeth are maxillary incisors (Borssen and Holm, 1997; Zaragoza et al., 1998).[16],[17] Radiographic examination of individuals who have undergone orthodontic treatment shows some loss of root length in 48% of cases (Remington et al., 1989)[19]. Radiographically clearly visible apical root resorption (> 2 mm to < 1/3 of root length) has been reported in 7% to 16% of patients treated with a fixed appliance (Hollender et al., 1980; Remington et al., 1989; Linge and Linge, 1991), whereas extreme root resorption leading to loss of more than one-third of the root length is seen in 1% to 0.4% (Remington et al., 1989; Janson et al., 2000).[18],[19],[20]
The etiology of root resorption during orthodontic treatment is multifactorial: type and duration of treatment, individual susceptibility, genetic predisposition, and systemic factors all may contribute. This resorptive potential varies between different patients and between different teeth in the same patient. Individual biologic factors, e.g., alveolar bone density, vascularity, and tooth structure may explain these variations (Melsen, 1999). [21]
Satu Apajalahti et al in 2002 studied a random sample of existing panoramic radiographs of 2000 university students for SRA. Roots as long as or shorter than the crowns in the incisors and visually evaluated as very short, blunt roots bilaterally in the posterior teeth were classified as SRA. The prevalence was 1.3%. According to anamnestic information, half the SRA patients had undergone orthodontic therapy, but pre-treatment radiographs were unavailable. In 70% of the SRA patients the short-rooted tooth pairs were upper incisors, but also involved were maxillary premolars, lateral incisors, and lower second premolars. Women were significantly more often affected.[6]
Maxillary incisors have been regarded as the most sensitive to root resorption (Newman, 1975; Remington et al., 1989), in particular those with blunt or pipette-shaped roots[10],[19]. Mandibular incisors and mandibular first molars are also more likely to lose root length than are other teeth (Kennedy et al., 1983).[22]
Some conditions of the dentition, dental anomalies, and morphological characteristics of the permanent dentition is mentioned as predisposing factors. These include hypodontia, invaginations, ectopic canines, atypical root resorption in connection with eruption of a permanent tooth, previous trauma, taurodontic molars, and SRA (Lind, 1972; Newman, 1975; Linge and Linge, 1991.[1],[9],[20]
Most studies have found no significant relationship with sex (reviewed by Brezniak and Wasserstein, 2002)[23], although, controversially, studies have shown a greater prevalence of root resorption in girls (Newman, 1975).[9]
Human Conditions Displaying Root-length Variation In 47, XXY males with an extra X chromosome, root development is affected, leading to the development of taurodontism (Varrela and Alvesalo, 1988). X-chromosome deficiency also appears to influence root formation.[24]
In Turner syndrome (45, X), root length and crown height of incisors, canines, and premolars are significantly reduced, and a complex root morphology in premolars and molars is a common finding (Midtbo and Halse, 1994).
Defective root formation is a characteristic feature in type I dentin dysplasia (I DD), a rare autosomal dominant disorder of the dentin (Shields et al., 1973)[25]. An epithelial defect has been suggested to underlie type I DD (Witkop, 1988); however, the gene mutation behind type I DD is thus far unknown. The affected teeth are clinically normal, but because of their short or almost missing roots, the teeth move easily, and spontaneous exfoliation may occur. In addition to short tapering roots, characteristic dental features are periapical radiolucencies and pulpal obliteration with crescent-shaped radiolucent pulp remnants in the permanent teeth[26]. Total pulpal obliteration is seen in the primary dentition (Steidler et al., 1984; Shankly et al., 1999). Histologically, the enamel and the outermost layer of the coronal dentin are normal, but the pulp chamber is obliterated by abnormal dentin[27],[28].
In taurodontic molars, the bifurcation area is located more apically than in normal molars, resulting in a proportionately shortened root and enlargement of the pulp chamber.[26]
Different prevalence figures have been reported for taurodontism, due to differences in diagnostic criteria. For taurodontic lower molars in one Dutch population, the prevalence was 10% (Schalk-van der Weide et al., 1993). Taurodontism has been associated with hypodontia (Seow and Lai, 1989; Arte et al., 2001) and Oligodontia (Schalk-van der Weide et al., 1993).[29]
Case Report:
A 14 year old boy was referred to the Pediatric Dental Clinic from a general dental practitioner with a chief complaint of mobility in the mandibular anterior teeth region. The mobility was evident in the mandibular anterior segment, due to which the patient was reluctant to have his teeth examined as he expressed the fear that he my spontaneously loose his teeth if anyone touched them (one of the reasons for the inability to take photographs).The patients as well as the parents revealed that the referring dentist had fixed the teeth with a wire upon their request.
The patient presented with two radiographs (one take at 11 years of age- Figure 1 & the other at 13.5 years of age - Figure 2).
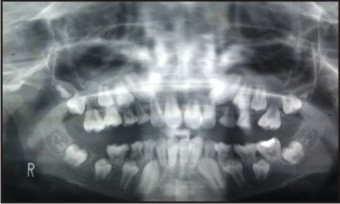 | Figure 1 - At 11 Years.
 |
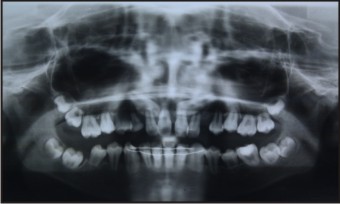 | Figure 2 - At 13.5 Years
 |
SRA is evident in both the radiographs (Figure 1 & 2) affecting the permanent dentition. Another unique Finding in this particular case is that the SRA seems to affect the primary teeth as well, especially the maxillary first primary molars bilaterally and the maxillary & mandibular second primary molars (65 having exfoliated) (Figure 1).
Apart from SRA, Taurodontism is also evident in the both the mandibular canines and the impaction of the permanent maxillary canines bilaterally (Figure 2)
Familial history was not significant with the parents or the sibling expressing any of the symptoms. Also the case was not associated with any syndrome or systemic anomaly (confirmed by routine blood examination and referral to a pediatrician)
The patient also presented with a fixed wire splint at the time of examination to stabilize the mobile mandibular anterior teeth (Evident in the radiograph).
After careful examination and study of this case & owing to the age, the patient has been kept under observation & appropriate treatment will be initiated once all the teeth erupt into the oral cavity.
Possible Treatment options being considered:
Option 1: Retain as many permanent teeth & provide a suitable prosthesis
Option 2: Orthodontically Correct the Maxillary canines after extracting the mobile teeth & utilizing the support provide a suitable restoration – Patient needs to be on long term follow up as literature has suggested that Orthodontic Treatment can induce SRA.
Option 3: Total Extraction Followed by implant supported dentures to improve the functionality & esthetics.
Discussion:
Teeth morphogenesis could be divided into two distinct and continuous phases - Cervical loop area is the stem cell niche and the germinal center of root development. When morphological changes occur in the cervical loop, degenerating from three layer (inner enamel epithelium, stratum intermedium and outer enamel epithelium) to two layer (inner and outer epithelium), teeth root growth is initiated. Certain specific genes may be what make a developmental choice in root or crown. BMP4 could be involved in the regulation of the epithelial stem cell niche. Notch’s and FGF10 are mainly absent, which could stop the growth and development of roots and maintain crown formation[30].
It is also suggested that BMPs, GDFs, MSXs and Amelogenin are involved in the earlier regulations of teeth root development. In addition, only one pathway regulating late tooth development has been identified, and that is the Ectodysplasin-A hormone signaling system including EDA, EDA receptor and nuclear factor I /transcription-replication proteins. Besides, NFI-C/CTF Transcription-Replication Factor knockout mice have unique tooth pathology of molars lacking roots, which prove that it plays an essential role in teeth root development.
But the regulatory mechanism needs further exploration. Moreover, screening causative genes of HTR disease will help our molecular understanding of master regulator in root formation.
The present case reports a non Syndromic occurrence of Short Root Anomaly (SRA).what makes this condition rare is its involvement of most of the permanent teeth and signs of its occurrence in the Primary Dentition as well. This condition is also associated with Taurodontism and Impaction of the mandibular & maxillary canine’s respectively. The literature is replete with cases where the SRA has mostly affected the Central incisors and the molars & this may be the result of orthodontic therapy or any genetic condition. [31],[32],[33],[34],[35],[36]
This particular case is of significance owing to the fact that this is a spontaneous occurrence of the condition with no predisposing factors or systemic conditions.
Conclusion:
Short root anomaly is a rare clinical condition. Generalized short root anomaly not associated with any other syndromes or systemic illness is even rarer. The present article presents such a case with a comprehensive review of reported cases, highlighting the probable etiological factors. Thorough Clinical, Radiographic & Genetic analysis of such conditions is extremely important, firstly to identify & then initiate appropriate interventions to improve the oral health of the person suffering from SRA.
What this paper adds:
1. This is the first reported case of SRA where most of the permanent Dentition along with taurodontism & impaction of Permanent Canines
2. This is also a significant case as is not associated with any syndromes or familial history.
3. Multidisciplinary treatment approach to improve the esthetics & functionality for the patient
References:
1. Lind V. Short root anomaly. Scandinavian Journal of Dental Research, 1972, 80:85-93.
2. Ando S, Kiyokawa K, Nakashima T, Shinbo K, Sanka Y, Oshima S, et al. Studies on the consecutive survey of succedaneous and permanent dentition in the Japanese children. Part 4.Behavior of short-rooted teeth in the upper bilateral central incisors. Journal Nihon University School of Dentistry, 1967, 9:67-80.
3. Jakobsson R, Lind V. Variations in root length of the permanent maxillary central incisor. Scandinavian Journal of Dental Research, 1973, 81:335-338.
4. Bergström K. An orthopantomographic study of hypodontia, supernumeraries and Other anomalies in school children between the ages of 8-9 years. Swedish Dental Journal, 1977, 1:145-157.
5. Brook AH, Holt RD. The relationship of crown length to root length in permanent maxillary central incisors. Proceedings of British Paedodontic Society, 1978, 8:17-20.
6. Apajalahti, S., Holtta, P., Turtola, L., et al. Prevalence of short-root anomaly in healthy young adults. Acta odontologica Scandinavica, 2002, 60(1): 56-59.
7. Apajalahti, S., Arte, S., Pirinen, S. Short root anomaly in families and its association with other dental anomalies. European Journal of Oral Sciences, 1999, 107(2): 97-101.
8. Brown, H. Hypoplasia of the dentition. American Journal of Orthodontics & Oral Surgery, 1944, 30(1): 102-103.
9. Newman WG.Possible etiologic factors in external root resorption. American Journal of Orthodontics, 1975, 67:522-539.
10. Lerman RL, Gold R. Idiopathic short root anomaly. Journal of Pedodontics, 1977, 4:327-333.
11. Brook AH, Holt RD. The relationship of crown length to root length in permanent maxillary central incisors. Proceedings of British Paedodontic Society, 1978, 8:17-20.
12. Edwards DM, Roberts GJ. Short root anomaly. British Dental Journal, 1990, 169:292-293.
13. Desai RS, Vanaki SS, Puranik RS, Rashmi GS, Nidawani P. An unusual combination of idiopathic generalized short-root anomaly associated with microdontia, taurodontia, multiple Dens invaginatus obliterated pulp chambers and infected cyst: a case report. Journal of Oral Pathology & Medicine, 2006 Aug; 35(7):407-9.
14. Rajić Šikanjić. A Case of Short-Root Anomaly in a Female from Medieval Istria. International Journal of Osteoarchaeology (1047-482X) 16 (2006), 2; 177-180
15. Saini TS, Aberrant root formation: review of root genesis and three case reports.Pediatric Dentistry, 2004 May-Jun; 26(3):261-5.
16. Borssen E, Holm AK. Traumatic dental injuries in a cohort of 16-year-olds in northern Sweden. Endodontics & Dental Traumatology, 1997, 13:276-280.
17. Zaragoza AA, Catala M, Colmena ML, Valdemoro C. Dental trauma in School children six to twelve years of age. ASDC Journal of Dentistry for Children, 1998, 65:492-444.
18. Remington DN, Joondeph DR, Årtun J, Riedel RA, Chapko MK. Long-term evaluation of root resorption occurring during orthodontic treatment. American Journal of Orthodontics & Dentofacial Orthopaedics, 1989, 96:43-46.
19. Hollender L, Rönnerman A, Thilander B. Root resorption, marginal bone support. and clinical crown length in orthodontically treated patients. European Journal of Orthodontics, 1980, 2:197-205.
20. Linge L, Linge BO. Patient characteristics and treatment variables associated with apical root resorption during orthodontic treatment. American Journal of Orthodontics & Dentofacial Orthopaedics, 1991, 99:35-43.
21. Melsen B. Biological reaction of alveolar bone to orthodontic tooth movement. Angle Orthodontics, 1999, 69:151-158.
22. Kennedy DB, Joondeph DR, Osterberg SK, Little RM. The effect of extraction and orthodontic treatment on dentoalveolar support. American Journal of Orthodontics, 1983, 84: 183-190.
23. Brezniak N, Wasserstein A. Orthodontically induced inflammatory root resorption. Part II: The clinical aspects. Angle Orthodontics, 2002, 72: 180-184. Review
24. Varrela J, Alvesalo L. Taurodontism in 47, XXY males: An effect of the extra X chromosome on root development. Journal of Dental Research, 1988, 67:501-502.
25. Shields ED, Bixler D, El-Kafrawy AM. A proposed classification for heritable human dentin defect with a description of a new entity. Archives of Oral Biology, 1973, 18:543-553.
26. Witkop CJ. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. Journal of Oral Pathology, 1988, 17:547-553. Review.
27. Steidler NE, Radden BG, Reade PC. Dentinal dysplasia: a clinicopathological study of eight cases and review of the literature. British Journal of Oral & Maxillofacial Surgery, 1984, 22:174-286.
28. Shankly PE, Mackie IC, Sloan P. Dentinal Dysplasia type I: report of a case. International Journal of Pediatric Dentistry, 1999, 9:37-42.
29. Schalk-van der Weide Y, Steen WHA, Bosman F. Taurodontism and length of teeth in patients with Oligodontia. Journal of Oral Rehabilitation, 1993, 20:401-412.
30. Tummers, M., Thesleff, I. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development, 2003, 130(6): 1049-1057
31. Viswanathan, H.L., Berry, J.E., Foster, B.L., et al. Amelogenin: A potential regulator of Cementum-associated genes. Journal of Periodontology, 2003, 74(10): 1423-1431.
32. Yamashiro, T., Tummers, M., Thesleff, I. expression of bone morphogenetic proteins and MSX genes during root formation. Journal of Dental Research, 2003, 82(3): 172-178.
33. Tucker, A.S., Headon, D.J., Schneider, P., et al. EDAR/EDA interactions regulate enamel knot formation in tooth morphogenesis. Development, 2000, 127(21): 4691-4700.
34. Shimomura, Y., Sato, N., Miyashita, A., et al. A rare case of hypohidrotic ectodermal Dysplasia caused by compound heterozygous mutations in the EDAR gene. Journal of Investigative Dermatology, 2004, 123(4): 649-655.
35. Tucker, A.S., Headon, D.J., Courtney, J.M., et al. The activation level of the TNF family receptor, EDAR, determines cusp number and tooth number during tooth development. Developmental Biology, 2004, 268(1): 185-194.
36. Steele-Perkins, G., Butz, K.G., Lyons, G.E., et al. Essential role for NFI-C/CTF transcription- replication factor in tooth root development. Molecular Cell Biology, 2003, 23(3): 1075-1084.
|