Introduction
The quest to live a long and healthy life is as old as the appearance of the first cell on earth. This cell replicated, multiplied, sustained and slowly developed into a very complex system called as the human body. Still, cell is the basic unit of life. Our body consists of mainly two kinds of cells; the ones which when damaged or lost cannot undergo the process of repair or regeneration. Any damage to these cells, either due to aging or injury may pose a threat to the whole system and thus has long been a concern to the mankind. On the contrary, it has also been observed that several tissues in the body (such as blood, skin, and gastrointestinal tract) undergo rapid renewal, and have regenerative ability .This observation lead the scientists to hypothesize that the tissues with the regenerative potential may contain cells that initiate their replacement. These cells are termed as “stem cells”. Stem cells are thus, the pioneer of regenerative medicine.[1]
A stem cell is a cell that has the ability to divide (self replicate) for indefinite periods—often throughout the life of the organism. Under the right conditions, or given the right signals, stem cells can give rise (differentiate) to the many different cell types that make up the organism.[2] Scientists primarily work with two kinds of stem cells from animals and humans: embryonic stem cells and adult stem cells.
The role of stem cells in the field of medicine for regenerating and repairing various parts of human body like heart , muscles , neuronal cells etc. has now been established.[3] Stem cells have also made their landmark into the field of dentistry with an anticipation to treat various oro-facial problems, which have high impact not only on the facial appearance, but also on quality of life- specifically on the ability to chew, a function that is easily taken for granted until lost. Combined with tissue engineering techniques, it is possible that dental stem cells may be used to engineer a complete tooth one day.[4]
The purpose of this article is to give the dental health practitioners a prior insight of the unprecedented opportunities of oral and tooth tissue regeneration, which though, have not reached the clinical set up today, but may become a norm in future, in the practice of every dental surgeon.
History
In 1878 first attempts were made to fertilize mammalian eggs outside the body. The term "stem cell" was proposed for scientific use by the Russian histologist Alexander Maksimov (1874–1928) in 1908. However, it took 60 years after that to accomplish in vitro fertilization of first human egg. It was in 1981 when scientists Martin Evans & Matthew Kaufman derived Mouse ES cells from the inner mass of blastocysts and grew them successfully in vitro. Following this, in 1994, Human blastocysts were generated and the inner cell mass was maintained in culture. ES like cells were formed in the center and retained stem cell like morphology. In 2003, Dr. Songtao Shi discovered new source of adult stem cells in primary teeth. In 2005, researchers at Kingston University in England claim discovered a third category of stem cell, dubbed cord-blood-derived embryonic-like stem cells (CBEs), derived from umbilical cord blood. The group claims these cells are able to differentiate into more types of tissue than adult stem cells. In January 2008, Human embryonic stem cell lines were generated without destruction of the embryo. Sabine Conrad and colleagues at Tübingen, Germany in October,2008 generated pluripotent stem cells from spermatogonial cells of adult human testis by culturing the cells in vitro under leukemia inhibitory factor (LIF) supplementation. On 30th October 2008, Embryonic-like stem cells were derived from a single human hair. On 28th May,2009, Kim et al. announced that they had devised a way to manipulate skin cells to create patient specific "induced pluripotent stem cells" (iPS), claiming it to be the 'ultimate stem cell solution'.The research is though endless and ever growing.
What Is Stem Cell ?
A stem cell is a special kind of cell that has a unique capacity to renew itself and to give rise to specialized cell types. Although most cells of the body, such as, heart cells or skin cells are committed to conduct a specific function, a stem cell is uncommitted and remains as such, until it receives a signal to develop into a specialized cell.[5] Their proliferative capacity combined with the ability to become specialized makes stem cells unique.
There are three defining features of a stem cell
1. Self-renewal = extensive proliferation: The abilityto self-renew has been linked conceptually to a stem cell’sability to divide extensively to form a vast numbers of cells.However, a stem cell is not immortal, but is endowed with acertain restricted capacity to self-renew related to how fasta tissue turns over.
2. Clonogenicity = stemness: A stemcell is thought tobe "clonogenic," which means that it canproliferate to forma colony of cells. However, whileclonogenicity ispart of the essential assay in defining a stemcell (that is,a single cell capable of proliferating and formingmultiplecell types), not all cells that form colonies qualifyas stemcells.
3. Stemness = undifferentiation: In manycases, a stemcell is thought to be an undifferentiated celltype (that is,it does not have a mature phenotype), but thereare instancesin which a cell with differentiated charactercan behave asa stem cell.
Classification of Stem cells
1) According to Stem cell Plasticity[6]
Totipotent Stem Cells: Cells that are capable of forming a completely new embryo that can develop into a new organism are called totipotent. A fertilized egg is totipotent. None of the stem cells used in research appear to have this capacity.
Pluripotent Stem Cells: Stem cells that have the potential to develop into any of the cell types found in an adult organism are called pluripotent. Embryonic stem cells are pluripotent.
Multipotent Stem Cells: Stems cells that only have the potential to make a few cell types in the body are called multipotent. Adult stem cells appear to be multipotent.
2) According to Stem cell Growth Stage[6]
Embryonic Stem Cells: Located within the inner cell mass of blastocyst stage of development.
Adult Stem Cells: Cells that have been isolated from various tissues including bone marrow, neural tissue, dental pulp and periodontal ligament.
3) According to Stem cell sources[7]
Autologous Stem Cells: Cells are obtained from the same individual in whom they will be implanted.
Allogenic Stem Cells: Cells originate from a donar of the same species.
Xenogenic Cells: Cells that are those isolated from individuals of another species.
Discussion
1) Embryonic Stem Cells: The first documentation of the isolation of embryonic stem cells from human blastocysts was in 1994.[8] Since then, techniques for deriving and culturing human ES cells have been refined.
Specifically, embryonic stem cells are derived from embryos that develop from eggs that have been fertilized in clinic and then donated for research purposes with informed consent of the donors. They are not derived from eggs fertilized in a woman's body. The embryos from which human embryonic stem cellsare derived are typically four or five days old and are a hollow microscopic ball of cells called the blastocyst.The blastocyst includes three structures: the trophoblast, which is the layer of cells that surrounds the blastocyst; the blastocoel, which is the hollow cavity inside the blastocyst; and the inner cell mass,which is a group of approximately 30 cells at one end of the blastocoel.[8] Figure1.
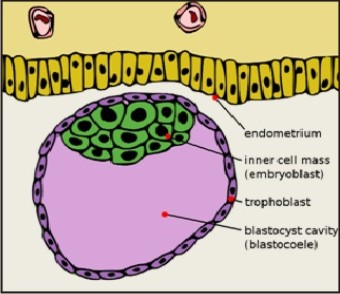 | Figure 1: Blastocyst Showing Inner Cell Mass And Trophectoderm
 |
Differentiation of embryonic stem cells
As long as the embryonic stem cells in culture are grown under certain conditions, they can remain undifferentiated (unspecialized). But if cells are allowed to clump together to form embryoid bodies,they begin to differentiate spontaneously in the presence of certain growth factors (like activin, retinoic acid, sonic hedgehog etc). They can form muscle cells, nerve cells, and many other cell types. Although spontaneous differentiation is a good indication that a culture of embryonic stem cells is healthy, it is not an efficient way to produce cultures of specific cell types.
Possible sources of Embroynic stem cells:
Embryos created via IVF (for infertility treatment or for research purposes), Embryos or fetuses obtained through elective abortion, Embryos created via SCNT (somatic cell nuclear transfer, or cloning)[8]
Potential Uses of Human Embryonic Stem Cells:
Many uses have been proposed for human embryonic stem cells. The most-often discussed is their potential use in transplant therapy - i.e., to replace or restore tissue that has been damaged by disease or injury.[8],[9]
To study early events in human development.
Used to explore the effects of chromosomal abnormalities in early development. This might include the ability to monitor the development of early childhood tumors, many of which are embryonic in origin
Human Embryonic Stem cells could also be used to test candidate therapeutic drugs.
Human Embryonic Stem cells could be employed to screen potential toxins.
Finally, human Embryonic Stem cells could be used to develop new methods for genetic engineering.
Advantages of using embryonic stem cells for transplant therapy:
Compared to adult stem cells ES cells have an unlimited ability to proliferate in vitro, and are more likely to be able to generate a broad range of cell types through directed differentiation.[8]
Disadvantages of the using of human ES cells for transplant therapy:
The propensity of undifferentiated ES cells to induce the formation of tumors (teratomas), which are typically benign.
2) Adult Stem Cells
Some scientists now use the term somatic stem cellinstead of adult stem cell. The adult stem cell are clonogenic & capable of self-renewal for the lifetime of the organism and give rise to fully differentiated cells that have mature phenotypes, are fully integrated into the tissue, and are capable of specialized functions that are appropriate for the tissue.[10] The following are examples of differentiation pathways of adult stem cells: (Figure 2)
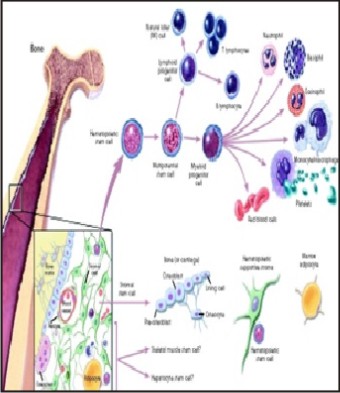 | Figure 2: Haematopoietic And Stromal Cell Differentiation
 |
Hematopoietic stem cells give rise to all types of blood cells: red blood cells, B lymphocytes, T lymphocytes, natural killer cells, neutrophils, basophils, eosinophils, monocytes, macrophages, and platelets.
Bone marrow stromal cells (mesenchymal stem cells) give rise to a variety of cell types: bone cells (osteocytes), cartilage cells (chondrocytes), fat cells (adipocytes), and other kinds of connective tissue cells such as those in tendons.
Neural stem cellsin the brain give rise to its three major cell types: nerve cells (neurons) and two categories of non-neuronal cells—astrocytes and oligodendrocytes.
Epithelial stem cells in the lining of the digestive tract occur in deep crypts and give rise to several cell types: absorptive cells, goblet cells, Paneth cells, and enteroendocrine cells.
Adult stem cells may also exhibit the ability to form specialized cell types of other tissues, which is known as transdifferentiationor plasticity.[10],[11] The following are the examples of adult stem cell transdifferentiation
Hematopoietic stem cells may differentiate into: three major types of brain cells (neurons, oligodendrocytes, and astrocytes); skeletal muscle cells; cardiac muscle cells; and liver cells.
Bone marrow stromal cells may differentiate into: cardiac muscle cells and skeletal muscle cells.
Brain stem cells may differentiate into: blood cells and skeletal muscle cells.
Current research is aimed at determining the mechanisms that underlie adult stem cell plasticity. If such mechanisms can be identified and controlled, existing stem cells from a healthy tissue might be induced to repopulate and repair a diseased tissue. (Figure3)
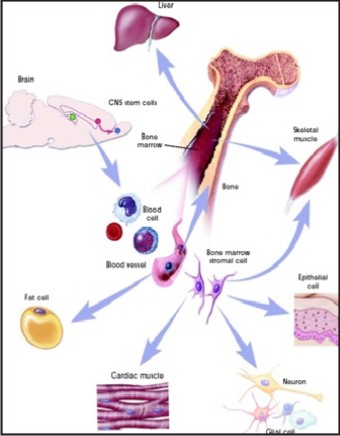 | Figure 3: Plasticity Of Adult Stem Cells
 |
Potential Uses of Adult Stem Cells:
1. Hematopoietic Stem Cell Rescue in Cancer Chemotherapy
2. Graft-Versus-Tumor Treatment of Cancer
3. Leukemia and Lymphoma
4. Inherited Blood Disorders
5. Other Applications in autoimmune diseases, such as diabetes, rheumatoid arthritis, and system lupus erythematosis, Parkinson’s disease.
Possible sources of adult stem cells:
Bone marrow- bone marrow stem cells, Peripheral blood- peripheral blood stem cells, Neurons- neuronal stem cells, Muscles- muscle stem cells, Liver- liver stem cells, Pancreas – pancreatic stem cells, Cornea and retina- corneal limbal stem cells, Mammary gland- mammary stem cells, Salivary glands, Skin- dermal hair follicle stem cells, Tendon, Synovial membrane, Heart, Cartilage, Thymic progenitors, Adipose tissue- adipose (fat) derived stem cells, Umbilical cord blood- cord blood stem cells, Amniotic stem cells, Blood vessels- mesangioblasts. [10],[11]
3) Dental Stem Cells
Possible sources of Dental adult stem cells:
Permanent teeth: Dental pulp stem cells (DPSCs): derived from third molar.[5]
Deciduous teeth: Stem cells from Human Exfoliated deciduous teeth- SHED: stem cells are present with in the pulp tissue of deciduous teeth.[13]
Human cementum derived cells- HCDC’s
Stem cells from supernumerary tooth- Mesiodens.[14]
Stem cells from teeth extracted for orthodontic purposes.[15]
Dental Follicle progenitor cells[16]
Stem cells from root apical papilla- SCAP[17]
Periodontal ligament : Periodontal ligament stem cells ( PDLSCs)[18]
Stem cells from human natal dental pulp.[19]
a) Dental Pulp Stem Cells (DPSCs)
Adult dental pulp stem cells (DPSCs) were discovered in wisdom teeth in 2000.[20] Researchers isolated Dental ectomesenchymal stem cells from the dental pulp of the extracted wisdom teeth.[21] Same as BMSC, DPSCs are colony forming plastic adherent cells which display very similar features.[22] Workers analyzed the profile of gene expression of DPSCs and BMSCs which show both cells are distinct precursor populations but have a very similar gene expression level.[23] In a chemically defined culture medium, DPSCs can be differentiated into smooth and skeletal muscle cells, neurons, and cartilage and bone cells.[24] The difference between BMSCs and DPSCs is DPSCs can differentiate into odontoblast like cells (dentin forming cells).[24] To determine the existence of DPSCs, previously developed methodology was used for the isolation and characterization of BMSCs and pluripotent postnatal stem cells. DPSCs were characterized as clonogenic and highly proliferative stem cells.[25] S. Gronthos et al. demonstrated that DPSCs possess all qualities of stem cells.[26] It is reported that DPSCs can differentiate into endothelial cells which can make functional blood carrying blood vessels.[27] Stem cells derived from the dental pulp can form pulp like tissue.[28] Pulp-like tissue could be engineered in vitro, using DPSCs seeded into synthetic matrices made with polyglycolic acid.[29] So as stem cells can differentiate in pulp like tissue and dentine pulp complex,[30] in future it is possible to replace infected pulp tissue of a paining tooth with newly generated pulp like tissue differentiated from stem cell and then patient will be without pain along with his vital teeth. Hence stem cell is topic of interest in discussion for regenerative endodontics. A big problem with dental implant is improper osteointegration which lead to implant failure but DPSCs have the ability to form bone that is useful for the osseointegration of dental implants coated with hydroxyapatite crystals, and may give good bone implant contact level.[31] Hence stem cell can increase the success rate of dental implants. Ming Yan et al. suggested that DPSCs are useful in reconstructing dentin pulp complex and biotooth.[30] Human tooth is made up of enamel, dentin, and cementum and pulp tissue. Enamel is formed by ameloblast cells, dentin is made by odntoblast cells, cementum is made by cementoblast cells. Stem cell can differentiate into all four tissues. Hence Ming Yan et al. suggested that Bio-tooth can be made from stem cells.[30] There are many studies which demonstrate that reconstruction of the biotooth is possible with dental stem cells.[28],[29],[30],[31],[32],[33]
b) SHED: stem cells from human exfoliated deciduous teeth
A population of high quality human stem cells was found in the exfoliated human primary teeth, recently (Miura et al, 2003).[13] Remnant dental pulp derived from exfoliated deciduous teeth contains a multipotent stem-cellpopulation. These stem cells can be isolated and expanded ex vivo,thereby providing a unique and accessible population of stem cellsfrom an unexpected tissue resource.
Previous experiments haveshown that dental pulp tissue of adult teeth contains a populationof DPSCs that are capable of differentiating into odontoblastsand adipocytes as well as expressing nestin and glial fibrillary acidic protein (GFAP) and forma dentin/pulp-like complex after in vivo transplantation.[13] Deciduous teeth are significantly different from permanent teethwith regards to their developmental processes, tissue structure,and function. Therefore, it is not a surprise to find that SHEDare distinct from DPSCs with respect to their higher proliferationrate, increased cell-population doublings, sphere-like cell-clusterformation, osteoinductive capacity in vivo, but failure to reconstitutea dentin-pulp-like complex , perhaps in order to have more immature characteristics than other post-natal stem cell population.[31]
The mechanisms controlling the growth and replacement of teeth are largely unknown, in particular with respect to howcraniofacial components including bone and soft tissues surroundingteeth participate in the process of tooth development. SHED demonstrateda strong capacity to induce recipient cell-mediated bone formationin vivo. SHED could not differentiatedirectly into osteoblasts but did induce new bone formation byforming an osteoinductive template to recruit murine host osteogeniccells.[31] These data imply that deciduous teeth may not only provideguidance for the eruption of permanent teeth, as generally assumed,but may also be involved in inducing bone formation during theeruption of permanentteeth.
It is notable that SHED expressed neuronal and glial cell markers, which may be related to the neural crest-cell origin ofthe dental pulp.[13] Neural crest cells play a pivotal rolein embryonic development, giving rise to a variety of cell typessuch as neural cells, pigment cells, smooth muscle, craniofacial cartilage, and bone. Studies demonstrated that BMSCs were also capable of differentiating into neural-like cellsafter in vivo transplantation. Dental pulp cells are knownto produce neurotrophic factors and even make the motor neurons survive afterspinal cord injury.[34]
Recent studies provide evidence that SHED represent a population of postnatal stem cells capable of extensive proliferation andmultipotential differentiation. Deciduous teeth therefore maybe an ideal resource of stem cells to repair damaged tooth structures,induce bone regeneration, and possibly to treat neural tissueinjury or degenerative diseases. However, the biological significanceof the existence of SHED remains to be determined.
c) Periodontal Ligament Stem Cells (PDLSCS)
The periodontium is a connective tissue organ which attaches the teeth with the bones of the jaws. It consists of periodontal ligament, gingiva, cementum and alveolar bone.[35] Human PDLSCs have been successfully isolated by scientists from the root of extracted teeth.[36],[37] Researchers demonstrated that if PDLSCs with hydroxyapatite (HA) or tricalcium phosphate (TCP) as a carrier are transplanted into immuno compromised mice, then it can be seen that PDLSCs have potentials of regenerating typical cementum and periodontal ligament like structure.[38] Studies suggest that if PDLSCs are transplanted directly into periodontal defect areas which are caused by periodontal disease, it might be a viable therapeutic approach.[39],[40] On the other hand, under in vitro conditions, PDLSCs display a low ability of differentiation into osteogenic tissue.[36] PDLSCs can get differentiated into cells or tissues that are very similar to periodontium.[36] Yi Liu, Ying Zheng, Ding et al. demonstrated the role of autologous PDLSCs to treat periodontitis in miniature pig preclinical model and their study indicated that a multilevel cellular or biomaterial treatment may be an optimal therapeutic approach for regeneration of periodontal tissue.[38] Researches have also isolated PDLSCs from pigs and sheeps.[41],[42] They also suggest that PDLSCs can successfully establish a functional periodontium.[41] Kawanabe et al. identified highly proliferating stem cells in human periodontal ligaments.[43] These demonstrations indicate that in future, tissue of the periodontium made by stem cell can be used as a treatment modality to replace the diseased periodontium around teeth so as to disappear mobility of tooth cause due to diseased periodontium. Many more studies are required for PDLSCs to provide new insights useful for regenerative therapy in dentistry.
d) Stem Cells from Apical Papilla (SCAP)
Researchers isolated stem cells from dental apical papilla of wisdom teeth or incisors of four months old mini pig termed as “Stem Cells from apical papilla”.[41],[44] Dental papilla is basically an embryonic tissue that is responsible for the formation of dental pulp and the crown. But SCAPs can only be isolated at certain specific stages of the development of tooth. As dental papilla contain higher number of adult stem cells than mature dental pulp, SCAPs have a greater potential for regenerating dentin than DPSCs.[41] SCAPs originate from an embryonic-like tissue so they are less likely to be differentiated than DPSCs. Study by Sonoyama W et al. demonstrate formation of dental connective tissue is induced by a combination of SCAPs and PDLSCs. But this study is not clear as to which stem cells were important for the synthesis of dental connective tissue.[41]
e) Dental Follicle Precursor Cells (DFPCs)
The dental follicle contains the precursors of the periodontium so it plays a very important role in development of tooth.[45] Cells of the dental sac develop into a mature periodontium which consists of alveolar bone, cementum and the periodontal ligaments (PDL).[45] Research workers have observed that Hertwig’s epithelial root sheath (HERS) disintegrates into epithelial fragments and allows contact between surface of dentin and dental follicle ectomesenchymal cells, and here these cells differentiate into mature cells of the periodontium.[46],[47] This demonstrate that dental follicles contain progenitor cells which have the capability of differentiating into cementum forming cells (cementoblasts), osteoblasts of the alveolar bone, and periodontal ligament fibroblasts. Handa K (2002) isolated progenitor cells from bovine dental follicles. In in vitro conditions these cells formed clusters of spheroid like cells and in in vivo conditions, cementum matrix formation took place by these cultured dental follicle cells.[48] The human dental follicle is a tissue which belongs to tooth germ, and after wisdom tooth extraction one can isolate these cells very easily. Ectomesenchymal cells are present in the dental follicles; these cells are derived from the neural crest. Similar to BMSCs, DFPCs are colony forming cells which are also plastic adherent. Under in vitro conditions these cells can be differentiated into osteoblast like cells.[49] Different workers suggest that like PDLSCs, DFPCs can also differentiate to produce mineralized tissue.[45],[50],[51] DFSCs can differentiate into mesenchymal derived cells like cementoblasts, adipocytes and chondrocytes.[49]
f) Human cementum derived cells- HCDC’s
Although there are differences in the organization of bone and cementum, it is not clear if they are formed by distinct cell types or by a bone-forming cell that has different environmental cues. Distinguishing between these two possibilities has been difficult because, till date, there is no specific marker for cementum or cementocytes.[52] Cultures of murine or primary human cementum-derived cells (HCDCs) have been established from healthy teeth using a collagenase pretreatment as had been established previously for the culture of trabecular bone cells. With primary human cementum-derived cells, discrete colonies that contained cells exhibiting fibroblast- like morphology are formed, and when the colonies became sufficiently large, cells from individual colonies were isolated and subcultured. Cementum-derived cells exhibit low levels or no alkaline phosphatase activity and mineralize in vitro to a lesser degree than Bone marrow stromal cell (BMSC) cultures. To study the differentiation capacities of HCDCs, cells were attached to hydroxyapatite/tricalcium phosphate ceramic and transplanted subcutaneously into immunocompromised mice.[52] Like individual colonies of human BMSCs, approximately 50 percent of the clonal HCDCs tested formed a bone-like tissue that featured osteocyte/cementocyte-like cells embedded within a mineralized matrix. However, the mineralized tissue was lined with a layer of cells that were somewhat more elongated than osteoblasts, and the HCDC matrix was somewhat less cellular than that produced by BMSCs. Unlike BMSC transplants, which developed lamellar bone, the HCDC matrix was found to contain unorganized collagen bundles, as is seen in cementum. Cells in the HCDC matrix were positive for fibromodulin and lumican, while osteocytes in the BMSC matrix were negative.[52] The HCDC transplants were also devoid of hematopoietic marrow. These results show that cells from normal human cementum can be isolated and expanded in vitro. Furthermore, these cells are capable of differentiating and forming a cementum-like tissue when transplanted into immunocompromised mice.
Diseases treated with stem cell therapy:
Virtually everyone could benefit from stem cell therapies whether directly, or indirectly. Across the world, stem cell transplants have been used since the 1960s to treat a variety of diseases.
Stem Cell Funding
Stem cell research, is not federally funded due to a ban placed on embryo research in 1995. Since then, this stem cell research has continued mainly through private funding. In early 1999, the NIH (National institutes of health, U.S. department of health and human services) announced that they would support research on embryonic stem cell lines that had already been previously established. This was a monumental step, as the potential benefits of stem cell research are very large, but in order to reap these benefits, a large and sustained research investment is needed. The federal government is the only realistic source of such large funds.[7]
Conclusion
In the face of extraordinary advances in the prevention, diagnosis, and treatment of human diseases, the inability of most tissues and organs, to repair and regenerate after damage is a problem that needs to be solved In the present scenario, science clearly indicates that the use of adult and embryonic stemcells for regeneration, reconstruction or repair isfeasible in principle. Regeneration of damaged periodontal tissue, bone, pulp, and dentin are problems that the dentist faces today. Stem cells present in dental pulp, periodontal ligament and alveolar bone marrow, have a potential to repair and regenerate tooth and periodontal structures. Substantial advances have been made to handle stem cells in the laboratory,and to exploit their inherent potential for the repair and regeneration. Translation of these advances into clinical practice is still a question that needs to be solved. How far this will succeed, however, depends on solving technical problemsthat still are significant. The next article in the series will give an insight on Stem cell markers, the potentialapplication of dental stem cells in craniofacial region and stem cell therapies in medicine to treat other systemic diseases, recruitment strategies for use in regeneration and tissue engineering, stem cell banking and future prospects.
References
1. Anderson, D.J., Gage, F.H., and Weissman, I.L.Can stem cells cross lineage boundaries? Nat. Med. 2001; 7, 393–395.
2. About I, Bottero MJ, de Denato P, Camps Jet al. Human dentin production in vitro. Exp Cell Res 2000; 258: 33-41.
3. Akintoye SO, Lam T, Shi Set al. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 2006; 38: 758-68.
4. Bluteau G, Luder HU, De Bari C et al.Stem Cells for tooth engineering. European Cells and Mat. 2008; 16: 1-9.
5. Alastair J. Sloan & Rachel J. Waddington. Dental pulp stem cells: what, where, how? International Journal of Paediatric Dentistry 2009; 19: 61–70.
6. Fortier LA.Stem cells: Clssification, Controversies, clinical applications. Vet Surg. 2005; 34: 415-23.
7. Marwara PP, Mani A Sachdev S, Sodhi NK, Anju A. Stem Cells in dentistry: an overview. Pravara Med Rev. 2012; 4(2).
8. Amit, M., Carpenter, M.K., Inokuma, M.S., Chiu, C.P., Harris, C.P., Waknitz, M.A., Itskovitz-Eldor, J., and Thomson, J.A.Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol.2000; 227, 271–278.
9. Bongso, A., Fong, C.Y., Ng, S.C., and Ratnam, S.Isolation and culture of inner cell mass cells from human blastocysts. Hum. Reprod.1994; 9, 2110–2117.
10. Ballas CB, Zielske SP, Gerson S.Adult bone marrow stem cell and gene therapies: implications for greater use. J Cell Biochemistry Supp 2002; 38: 20–8.
11. Jiang Y, Jahagirdhar BN, Reinhardt RL et al.Pleuripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418: 41-49.
12. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920-6.
13. Miura M, Gronthos S, Zhao M, Lu Bet al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 2003; 100(10): 5807–12.
14. Huang AH, Chen YK, Lin LM, Shieh TY, Chan AW.Isolation and characterization of dental pulp stem cells from a supernumerary tooth. J Oral Pathol Med. 2008;39: 571-4.
15. Yang XC, Fan MW.Identification and isolation of human dental pulp stem cells. Zhonghua Kou Qiang Yi Xue Za Zhi. 2005; 40: 244-7.
16. Huang GT, Gronthos S, Shi S.Mesenchymal stem cells derived from dental tissues versus those from other sources: their biology and role in regenrative medicine.J Dent Res 2009; 88: 792-806.
17. Sonoyama W, Lin Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Charactterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 2008; 34: 166-71.
18. Ballini A, De Frenza G, Cantore S, Papa F, Grano M, Mastrangelo F et al.In vitro stem cells cultures from human dental pulp and periodontal ligament: a new prospects in dentistry. Int J Immunopathol Pharmacol 2007; 20: 9-16.
19. Karaoz E, Dooan BN, Aksoy A, Gacar G, Akytiz S, Ayhan S, et al.Isolation and in vitro characterization of dental pulp stem cells from natal teeth. Histochem Cell Biol 2010; 133: 95-112.
20. Gronthos S, Mankani M, Brahim J, Robey PG et al.Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 2000;97: 13625-13630.
21. Luciano Casagrande ,Mabel M. Cordeiro,Silvia A. Nor Jacques E. Nor.Dental pulp stemcells in regenerative dentistry. Odontology. 2011;99:1–7.
22. Christian Morsczeck, Gottfried Schmalz, Torsten Eugen Reichert, Florian Völlner, Kerstin Galler, Oliver Driemel.Somatic stem cells for regenerative dentistry. Clin Oral Invest. 2008; 12:113–118.
23. Shi S, Granthos S.Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003; 18: 696-704.
24. Christian Morsczeck , Gottfried Schmalz ,Torsten Eugen Reichert , Florian Völlner, Kerstin Galler, Oliver Driemel.Somatic stem cells for regenerative dentistry. Clin Oral Invest. 2008; 12:113–118.
25. Gronthos S, Mankani M, Brahim J, Robey PG et al.Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 2000;97: 13625-13630.
26. Gronthos S, Brahim J, Li W, Fisher LWet al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81(8): 531–5.
27. Sakai VT, Zhang Z, Dong Z, Neiva K, Machado M, Shi S, Santos C, Nör JE.SHED differentiate into functional odontoblasts and endothelium. J Dent Res 2010; 89:791–6.
28. Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007; 13:151–157.
29. Buurma B, Gu K, Rutherford R.Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci. 1999; 107:282-289.
30. Ming Yan , Yan Yu , Guangdong Zhang , Chunbo Tang, Jinhua Yu.A Journey from Dental Pulp Stem Cells to a Bio-tooth.Stem Cell Rev and Rep 2011; 7:161–171.
31. Yamada, Y., Nakamura, S., Ito, K., Sugito, T., Yoshimi, R., Nagasaka, T., et al.. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng Part A. 2010 Jun;16(6):1891-900.
32. Hu, B., Nadiri, A., Kuchler-Bopp, S., Perrin- Schmitt, F., Peters, H., and Lesot, H.Tissue engineering of tooth crown, root, and periodontium. Tissue Engineering, 2006; 12: 2069–2075.
33. Honda, M. J., Tsuchiya, S., Sumita, Y., Sagara, H., and Ueda, M. The sequential seeding of epithelial and mesenchymal cells for tissue engineered tooth regeneration. Biomaterials. 2007; 28: 680–689
34. Seo, B. M., Sonoyama, W., Yamaza, T., Coppe, C., Kikuiri, T., Akiyama, K., et al.SHED repair critical-size calvarial defects in mice. Oral Diseases. 2008; 14: 428–434.
35. Melcher A . H et al.Cells of periodontium: their role in the healing of wounds. Ann R Coll Surg Engl. 1985 March; 67(2): 130–131.
36. Seo BM, Miura M, Gronthos S et al.Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004; 364(9429): 149–55.
37. Seo BM, Miura M, Sonoyama W, Coppe C et al. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res 2005; 84: 907-912.
38. Yi Liu, Ying Zheng, Gang Ding, Dianji Fang, Chunimei Zhang, Peter Mark, Bartold,Stan Gronthos, Songtao Shi, Songlin Wang.Periodontal Ligament Stem Cell-Mediated Treatment for Periodontitis in Miniature Swine. STEM CELLS 2008; 26:1065–1073.
39. Thesleff I, Tummers M.Stem cells and tissue engineering: Prospects for regenerating tissues in dental practice. Med Princ Pract 2003;12:43–50.
40. Bartold PM, Shi S, Gronthos S.Stem cells and periodontal regeneration. Periodontol 2006;40:164 –172.
41. Sonoyama W, Liu Y, Fang D et al.Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006; 1: e79.
42. Gronthos S, Mrozik K, Shi S, Bartold PM.Ovine periodontal ligament stem cells: isolation, characterization, and differentiation potential. Calcif Tissue Int. 2006; 79:310–317.
43. Kawanabe N, Murakami K, Takano-Yamamoto T.The presence of ABCG2-dependent side population cells in human periodontal ligaments. Biochem Biophys Res Commun.2006; 344:1278–1283.
44. Jo YY, Lee HJ, Kook SY, Choung HW, Park JY Chung JH, Choung YH, Kim ES, Yang HC, Choung PH.Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007; 13:767–773.
45. Morsczeck C, Gotz W, Schierholz J, Zeilhofer F et al.Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 2005; 24: 155-165.
46. Spouge JD.A new look at the rests of Malassez: a review of their embryological origin, anatomy, and possible role in periodontal health and disease. J Periodontol. 1980; 51:437–444.
47. McNeil RL, Thomas HF.Development of the murine periodontium II Role of the periodontal attachment. J Periodontol. 1993; 64:285–291.
48. Handa K, Saito M, Yamauchi M, Kiyono T, Sato S, Teranaka T, Narayanan SA.Cementum matrix formation in vivo by cultured dental follicle cells. Bone . 2002; 31:606–611.
49. Christian Morsczeck , Gottfried Schmalz , Torsten Eugen Reichert , Florian Völlner, Kerstin Galler, Oliver Driemel.Somatic stem cells for regenerative dentistry. Clin Oral Invest. 2008; 12:113–118.
50. Morsczeck C, Moehl C, Gotz W, Heredia A, Schaffer TE, Eckstein N, Sippel C, Hoffmann KH.In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol Int. 2005; 29:567–575.
51. Morsczeck C.Gene expression of runx2, osterix, c-fos, DLX-3, DLX-5 and MSX-2 in dental follicle cells during osteogenic differentiation in vitro. Calcif Tissue Int. 2006; 78:98–102.
52. Shi S, Granthos S.Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003; 18: 696-704.
|