Clinical need
A tooth is a major organ and consists of multiple tissues. The hard tissues of the tooth include the enamel, dentin and cementum. The only vascularized tissue of the tooth is dental pulp that is encased in the mineralized dentin[1]. Life ends for a number of wildlife species after loss of complete dentition[2]. In humans, tooth loss can lead to physical and mental suffering that compromise an individual’s self-esteem and quality of life[3]. Americans make about 500 million visits to dentists each year. In 2009, an estimated $102 billion was spent on dental services in the U.S. Dental carries is one of the most common disorders in humans, second only to common cold. Dental caries, also known as tooth decay or cavity, is an infectious disease primarily by bacterial colonies that breakdown hard tissues of the tooth such as enamel and dentin, as well as soft tissue of the tooth known as dental pulp. According to CDC, 1 in 2 Americans are affected by tooth decay by age 15. By age 20, roughly 1 in 4 teeth are decayed or filled in the U.S. By age 60, more than 60% of the teeth and more 90% of the Americans are affected by dental caries.
Periodontal disease is another major cause for tooth loss. In both children and adults, facial trauma may also lead to tooth loss. Teeth can be congenitally missing, as a phenotype of myriads craniofacial anomalies including cleft palate[4],[5],[6],[7]. Resection of orofacial tumors may involve the extraction of teeth. Indeed, tooth loss represents a major challenge for contemporary dentistry or stomatology, and the bulk of daily dental practice.
Contemporary dentistry or stomatology restores mis- sing teeth by dentures or dental implants. Whereas dental implants are becoming favorite choices in developed countries, a large segment of the world population, frequently in developing countries, cannot afford dental implants. Dental implants, despite being the currently preferred treatment modality, can fail and will not adapt with surrounding bone that necessarily remodels through- out life[8]. A comparison of dental implants and regenerated teeth is provided in Table 1.
Dental profession has had the longstanding aspiration to regenerate teeth[9],[10],[11].
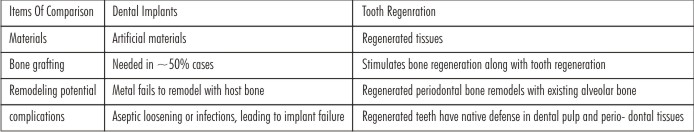 | Table 1 Comparison Of Current Dental Treatments Including Dental Implants And Dentures With Tooth Regeneration
 |
Regeneration of teeth can be broadly divided into several areas as listed below. References and review articles are provided for those areas that are not covered in this article:
Regeneration or de novo formation of the entire, anatomically correct teeth (discussed at length below; c.f. [12];
Regeneration of dental pulp (discussed below; c.f. [13]);
Regeneration of dentin based on biological approaches and potentially as biological fillers that may replace current synthetic materials for restorative dentistry[14],[15],[16];
Regeneration of cementum as a part of periodontium regeneration or for loss of cementum and/or dentin resulting from trauma or orthodontic tooth movement[17],[18];
Regeneration of the periodontium including cementum, periodontal ligament and alveolar bone[19],[20],[21],[22];
Regeneration or synthesis of enamel-like structures that may be used as biological substitute for lost enamel[23],[24],[25].
Since a tooth is a biological organ, it is unavoidable that regeneration of various components of the tooth is highly inter-connected. Furthermore, successful regeneration of tooth components does not necessarily translate to regeneration of an entire tooth. The overall objective of this review article is to explore therapeutically viable approaches for tooth regeneration by contrasting cell transplantation and cell homing approaches.
A new concept: brand new teeth made in the laboratory
The rapid progress made in stem cell, material and molecular biology sciences over the last 20 years has allowed scientists working on teeth to imagine alternative and innovative strategies for tooth replacement[3],[4]. Recently, scientists have implemented a new concept of tooth replacement, where new whole teeth could be generated experimentally using stem cells (referred to as BioTeeth, meaning living teeth)[3]. Regenerated tooth (RegTooth) is a more appropriate term, since it is possible to distinguish between naturally and experimentally formed teeth. The rationale for the generation of a new whole tooth is simple and consists of recreating and mimicking the molecular and cellular events that occur during the initiation of odontogenesis. This procedure might be a better alternative to the use of dental implants for tooth replacement, since it involves the regrowth and eruption of new teeth in the mouth of the patients, after experimental manipulation in vitro. However, a regenerated tooth has several challenges that need to be solved prior to any clinical trial/application. The reactivation of the odontogenic program using stem cells is not obvious and does not guarantee the success of new tooth formation in an adult mouth. It is possible to regenerate several human dental tissues (e.g., dentin and PDL) after experimental manipulation[3],[4],[5]. However, the regenerated tissues were not identical to their naturally formed counterparts. With the continuous progress of science it might be possible in the future to regenerate more complex dental structures (e.g., enamel) or even the entire tooth. Scientists working in that field are confronted daily with new challenges and limitations that might postpone the generation of brand new teeth in the laboratory for many years.
Barriers of tooth regeneration towards clinical appli- cations
For the regeneration of the entire tooth or tooth elements, we are ingrained to believe that stem cells and/or other cells must be transplanted. When tissue engineering was initiated as an interdisciplinary approach to heal tissue defects, three key components were pro- posed: cells, biomaterial scaffolds and signaling factors[26]. There is no question that cells, including stem/ progenitor cells, play central roles in tissue regeneration. However, do cells (including stem/progenitor cells) necessarily need to be taken out of the body, manipulated ex vivo and transplanted back into the patient?
Tooth regeneration by cell transplantation is a meritorious approach. However, there are hurdles in the translation of cell-delivery-based tooth regeneration into therapeutics. The most important one of these difficulties is inaccessibility of autologous embryonic tooth germ cells for human applications[9],[27],[28]. Xenogenic embryonic tooth germ cells (from non-human species) may elicit immunorejection and tooth dysmorphogenesis. Autologous postnatal tooth germ cells (e.g. third molars) or autologous dental pulp stem cells are of limited availability and remain uncertain as a cell source to regenerate an entire tooth. Regardless of cell source, cell-delivery approaches for tooth regeneration, similar to cell-based therapies for other tissues, encounter translational barriers. The costs of commercialization process and difficulties in regulatory approval in association with ex vivo cell manipulation have precluded any significant clinical translation effort to date in tooth regeneration (Table 2). As in tissue engineering of other biological structures, regeneration of an entire tooth or various tooth structure, including the enamel, dentin, cementum and dental pulp, by cell transplantation encounters a number of scientific, translational and regulatory difficulties[29].
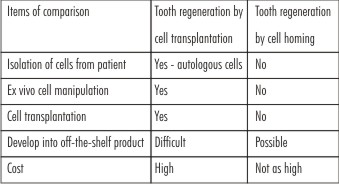 | Table 2 Comparison Of Cell Transplantation Vs. Cell Homing Approaches For Tooth Regeneration:
 |
Tooth regeneration by cell transplantation
Disassociated cells of porcine or rat tooth buds in biomaterials yielded putative dentin and enamel organ[30],[31]. Tooth bud cells and bone marrow osteoprogenitor cells in collagen, PLGA or silk-protein scaffolds induced putative tooth-like tissues, alveolar bone and periodontal ligament[32],[33],[34]. Embryonic oral epithelium and adult mesen- chyme together up-regulate odontogenesis genes upon mutual induction, and yielded dental structures upon transplantation into adult renal capsules or jaw bone[35]. Similarly, implantation of E14.5 rat molar rudiments into adult mouse maxilla produced tooth-like structures with surrounding bone[9],[36]. Multipotent cells of the tooth apical papilla in tricalcium phosphate in minipig incisor extraction sockets generated soft and mineralized tissues of the root[37]. Dental bud cells from unerupted molar tooth of a 1.5-month-old swine were expanded and then seeded in gelatin-chrondroitin-hyaluronan-tri-copolymer scaffold. Cell-seeded scaffolds were implanted autologously in the swine’s tooth extraction socket. Thirty-six weeks after implantation, dentin/pulp-like complex structures were identified with odontoblast-like cells and blood vessels in the pulp and appearance of cellular cementum[34]. However, the regenerated teeth were much smaller in size than the normal teeth in the same host.
E14.5 oral epithelium and dental mesenchyme were reconstituted in collagen gels and cultured ex vivo[27], and when implanted into the maxillary molar extraction sockets in 5-week-old mice, tooth morpho- genesis took place and was followed by eruption into occlusion[28]. Several studies have begun to tackle an obligatory task of scale up towards human tooth size[38],[39]. Thus, tooth regeneration by cell transplantation is a meritorious approach. However, there are hurdles in the translation of cell-delivery-based tooth regeneration into therapeutics. Autologous embryonic tooth germ cells are inaccessible for human applications[9],[27],[28]. Xenogenic embryonic tooth germ cells (from non- human species) may elicit immunorejection and tooth dysmorphogenesis. Autologous postnatal tooth germ cells (e.g. third molars) or autologous dental pulp stem cells are of limited availability. Regardless of cell source, cell delivery for tooth regeneration, similar to cell-based therapies for other tissues, encounters translational barriers. Excessive costs of commercialization and difficulties in regulatory approval have precluded, to date, any significant clinical translation of tooth regeneration. Dental pulp is a vascularized tissue encapsulated in highly mineralized structures including dentin, enamel and cementum, and maintains homeostasis of the tooth as a viable biological organ[40]. The overall health of the tooth is compromised upon dental pulp trauma or infections, frequently manifested as pulpitis. A typical endodontic treatment or root canal therapy for irreversible pulpitis is pulpectomy, involving pulp extirpation followed by root canal enlargement and obturation of root canal with gutta percha, a bioinert thermoplastic material. Despite reported clinical success, endodontically treated teeth become de-vitalized and brittle, susceptible to post-operative fracture and other complications inclu- ding re-infections due to coronal leakage or microleakage[41]. A substantial amount of tooth structures including enamel and dentin is removed during endodontic treatment, potentially leading to post-treatment tooth fracture and trauma[41],[42]. Endodontically treated teeth have lost pulpal sensation, and are deprived of the ability to detect secondary infections[42],[43]. The complications of current endodontic treatment are inevitable because of pulp devitalization or the loss of the tooth’s innate homeostasis and defense mechanisms.
Similar to tooth regeneration, existing effort in dental pulp regeneration has focused on cell transplantation[44],[45],[46]. Several reports have documented regeneration of dental pulp-like tissue in vitro or ectopically by transplantation of dental pulp stem cells[47],[48],[49],[50]. Deciduous and adult dental pulp stem cells seeded in a self-assembling peptide-amphiphile hydrogel showed distinctive behavior: greater proliferative rate for deci- duous cells but greater osteogenic differentiation potential for adult cells[47],[48]. Delivery of collagen scaffolds with dental pulp stem cells and dentin matrix protein-1 in dentin slices in mice led to ectopic formation of pulp- like tissue[50]. Deciduous dental pulp stem/progenitor cells seeded in matrigel in 1.5-mm cross-sectional tooth slices regenerated vascular pulp-like tissue following ectopic implantation in SCID mice[51]. Similarly, stem/progenitor cells from apical papilla and dental pulp in root fragments yielded vascularized pulp-like tissue following ectopic implantation also in SCID mice[50]. Despite its scientific validity, dental pulp regeneration by dental pulp stem cells encounters clinical and commercialization hurdles. Pulpectomy, the most common endodontic treatment, involves extirpation of dental pulp, and therefore leaves no dental pulp stem cells in the same tooth for pulp regeneration. For a patient who requires endodontic treatment in a given tooth but has intact dentition otherwise, no healthy tooth is to be sacrificed for isolation of dental pulp stem cells. Even in patients whose autologous dental pulp stem cells can be harvested, e.g. from extracted wisdom teeth, clinical therapy of dental pulp regeneration is difficult to develop due to excessive costs including cell isolation, handling, storage, and shipping, ex vivo manipulation, immune rejection (for allogeneic cells), not to mention liabilities of potential contamination, pathogen trans-mission and tumorigenesis that may be associated with cell transplantation[52]. Regeneration of dental pulp is discussed in detail in Kim et al.[13]. A biomaterial tooth scaffold can be fabricated by 3D bioprinting (Figure 1). For a patient who needs to have a tooth extracted, anatomic form can be derived from CT or MRI scans of the contralateral tooth (if it is healthy) or published anatomic norms. Two-dimensional CT or MR images can be reconstructed to yield high resolution 3D shape and dimensions of the patient’s tooth to be extracted. The fabricated 3D tooth scaffold can be sterilized and shipped to the clinic within 2-3 days. Upon tooth extraction, the dentist implants the biomaterial tooth scaffold. In our report[12], a bio-root was regenerated within ~2 months. The advantage of this approach is that no stem cells need to be harvested or ex vivo manipulated.
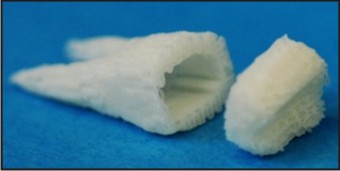 | Figure 1 :Biomaterial Scaffold Fabricated From The Patient’s Tooth
 |
Tooth regeneration by cell homing
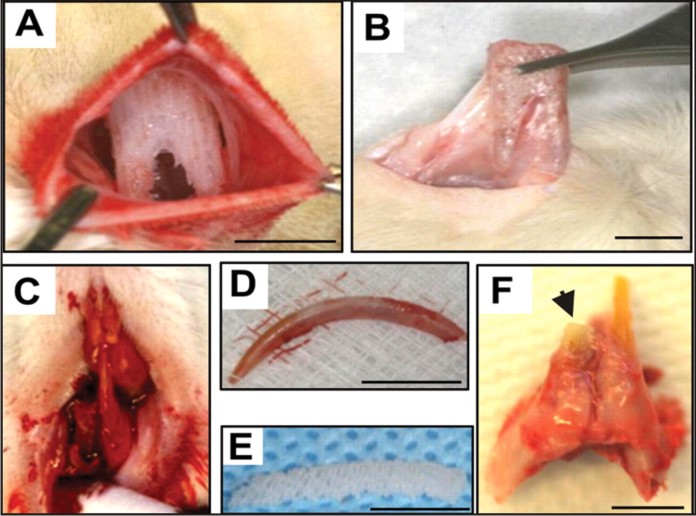
As an initial attempt to regenerate teeth, we first fabricated an anatomically shaped and dimensioned scaffold from biomaterials, using our previously reported approach[53],[54]. The dimensions of the permanent mandibular first molar were derived from textbook averages and therefore IRB exempt. Scaffolds with the shape of the human mandibular first molar (Figure 2A) were fabricated via 3D layer-by-layer apposition [54],[55]. The composite consisted of 80% (m/m) polycaprolactone (PCL) and 20% (m/m) of hydroxyapatite (HA) (Sigma, St. Louis, MO). PCL-HA was co-molten at 120 ¢J and dispensed through a 27-gauge metal nozzle to create repeating 3D microstrands (200 μm wall thickness) and interconnecting microchannels (dia: 200 μm) (Figure 2A).
All scaffolds were sterilized in ethylene oxide for 24 h. A blended cocktail of stromal derived factor 1 (SDF1) (100 ng.mL-1) and bone morphogenetic protein 7 (BMP7) (100 ng.mL-1) was adsorbed in 2 mg.mL-1 neutralized type ¢¹ collagen solution (all from R&D, Minneapolis, MN). SDF1 was selected for its effects to bind to CXCR4 receptors of multiple cell lineages including mesenchymal stem/progenitor cells[55],[56]. BMP7 was selected for its effects on dental pulp cells, fibroblasts and osteoblasts in elaborating mineralization [57],[58]. SDF1 and BMP7 doses were chosen from in vivo work[56],[59]. SDF1- and BMP7-loaded collagen solution was infused in scaffold’s microchannels by micropippeting, and crosslinked at 37 ¢J for 1 h. Control scaffolds were infused with the same collagen gel but without growth- factor delivery.
 | Figure 2
 |
Tooth regeneration by cell homing. (A) A 3D biomaterials scaffold was fabricated by layer by layer fabrication via bioprinting. In a clinical setting, a patient's missing tooth can be reconstructed by multi-slice imaging using CT or MRI of the contralateral, normal tooth or from anatomic averages. Microchannels are built in the 3D biomaterial human tooth shaped scaffold and serve as conduits for cell recruitment and vascularization. (B) Harvest of human shaped tooth scaffold following 9-week in vivo implantation. (C) A rat shaped tooth scaffold was implanted to replace the rat lower incisor that was freshly extracted. (D) Harvest of regenerated tooth scaffold showed the formation of multiple dental tissues including newly formed alveolar bone (ab), periodontal ligament-like tissue (pdl), dentin-like tissue (d) and dental pulp-like tissue (dp) with blood vessels (arrows).
A total of 22 male Sprague-Dawley rats (12-week-old) were randomly divided equally into treatment and control groups (Charles River, NY). All rats were anesthetized by i.p. administration of ketamine (80 mg.kg-1) and xylazine (5 mg.kg-1). A 2-cm incision was made in the dorsum. Human mandibular molar scaffolds were im- planted in surgically created subcutaneous pouches followed by wound closure. The rat right mandibular central incisor was extracted with periotome, followed by implantation of the anatomically shaped mandibular incisor scaffold[12] into the extraction socket. The flap was advanced for primary closure around the scaffold.
Nine weeks post-surgery, all rats were euthanized by pentobarbital overdose. The human shaped mandibular first molar scaffold was retrieved from the dorsum (Figure 2B). The rat incisor scaffolds were harvested with surrounding bone and native tooth structures (Figure 2C) (also c.f. [12]). All samples were fixed in 10% formalin, embedded in poly(methyl methacrylate) (PMMA), sectioned at 5-μm thickness for hematoxylin and eosin (H&E) and von-Kossa (VK) staining (HSRL, Jackson, VA). PMMA was used because PCL-HA sca- ffolds cannot be de-mineralized for paraffin embedding. The average areal cell density and blood vessel numbers were quantified from the coronal, middle, and apical thirds of the rat incisor scaffolds and similarly of the human molar scaffolds by a blinded and calibrated examiner. Microscopically, host cells populated scaffold’s micro- channels with growth-factor delivery (Figure 2D). Quan- titatively, combined SDF1 and BMP7 delivery homed significantly more cells into the microchannels of the human molar scaffolds than without growth-factor delivery (P<0.01)[12]. Angiogenesis took place in micro- channels with growth-factor delivery as exemplified in Figure 2D. Combined SDF1 and BMP7 delivery elabo- rated significantly more blood vessels than without growth-factor delivery (P<0.05)[12]. Scaffolds in the shape of the rat mandibular incisor integrated with surrounding tissue, showing tissue ingrowth into sca- ffold’s microchannels (Figure 2D). It was not possible to separate the implanted scaffolds without physical damage to surrounding tissue. Microscopically, the scaffolds within the extraction sockets clearly showed multiple tissue phenotypes including the newly formed alveolar bone (ab) and newly formed dentin-like tissue (d) with a fibrous tissue interface that is reminiscent of the perio- dontal ligament (pdl) in between (Figure 2D). There were areas of irregular cementum-like tissue (c) that did not completely cover dentin-like tissue (Figure 2D). Dental pulp (dp)-like tissue was formed in scaffold's microchannels and was rich with angiogenesis (Figure 2D). Quantitatively, combined SDF1 and BMP7 delivery elaborated significantly more blood vessels than growth- factor-free group (P<0.05) [12].
Anatomically dimensioned tooth scaffolds were desig- ned and 3D bioprinting was performed as follows: ana- tomic shape and dimensions of the rat mandibular central incisor and human mandibular first molar were derived from multiple slices of 2D laser scanning of extracted rat incisor and mandibular first molar. The anatomical contour of an extracted rat mandibular central incisor and human mandibular first molar was acquired from computed tomography scans and manipulated using computer aided design software (Rhinoceros, McNeel, Seattle, WA) for 3D reconstruction. Engineering parameters were used to fabricate a composite polymer scaffold per our prior methods[53],[54]. Scaf- folds with the shape of the human mandibular first molar (Figure 2A, 2B) were fabricated via 3D layer-by-layer apposition[53],[54]. The composite consisted of 80% (m/m) polycaprolactone (PCL) and 20% (m/m) of hydroxy- apatite (HA) (Sigma, St. Louis, MO). PCL-HA was co- molten at 120 ¢J and dispensed through a 27-gauge metal nozzle to create repeating 3D microstrands (20 μm wall thickness) and interconnecting microchannels (diameter: 200 μm) (Figure 2A). SDF1 is chemotactic and anti-apoptotic for bone marrow stem/progenitor cells and endothelial cells[55]. SDF1’s role to elaborate angiogenesis is likely of para- mount importance because stem/progenitor cells usually derive from via blood vessels or perivascular cells. Neovascularization in engineered teeth plays an important role in tissue survival, and promotes cell growth and mineralization[60]. SDF1 has effects to bind to CXCR4 receptors of multiple cell lineages including mesenchymal stem/progenitor cells. It binds to CXCR4, a chemokine receptor for endothelial cells and bone marrow stem/ progenitor cells[55],[56]. As an another cell homing factor BMP7 was chosen because it plays a key role in the differentiation of mesenchymal cells into osteoblasts[61]. BMP7 has many effects on dental pulp cells, fibro-blasts and osteoblasts in elaborating mineralization[58],[59]. BMP7 plays a key role in the differentiation of mesenchymal cells into osteoblasts[61] triggering the phosphorylation of SMAD1 and SMAD5, which in turn induces the transcription of many osteogenic/odonto- genic genes[62]. Along with variety of animal models, clinical trials investigating long bone applications have also provided supportive evidence for the use of BMP7 in the treat- ment of open many fractures and atrophic nonunions as well as in spinal fusion. BMP7 doses for cell homing approach for tooth regeneration were chosen from the promising therapeutic potential for this molecule from the positive clinical data[59]. SDF1 dose was chosen from an in vivo work showing SDF1 is induced in the periosteum of injured bone and promotes endochondral bone repair[56]. These findings represent the first report of the regeneration of tooth-like structures in vivo without cell delivery, and may provide a clinically translatable app- roach. Interconnecting microchannels (diameter: 200 μm) were constructed as conduits within anatomically correct scaffolds to allow the homing of host endogenous cells and angiogenesis. Upon in vivo implantation, a putative periodontal ligament and new bone formed at the sca- ffold’s interface with native alveolar bone. Remarkably, cell homing mediated by a cocktail of SDF1 and BMP7 recruited not only more cells, but also elaborated more vasculature throughout the scaffold’s microchannels than without growth-factor delivery.
Cell homing offers an alternative, especially regarding clinical translation, to previous meritorious methods of tooth regeneration by cell transplantation. The omission of cell isolation and ex vivo cell manipulation accelerates regulatory, commercialization and clinical processes[63]. The cost of cell-homing-based tooth regeneration is not anticipated to be as robust as cell delivery with regard to both commercialization process and as a treatment cost to the patient. Cell homing is an under-recognized app- roach in tissue regeneration[52]. Here, all cells in growth-factor delivery or growth-factor-free scaffolds are host derived endogenous cells. Tissue genesis requires condensation of sufficient cells of correct lineages[9],[64]. The observed putative periodontal ligament and adjacent, newly formed bone suggest the potential of combined delivery of SDF1 and BMP7 to recruit multiple cell lineages. Additional growth factors may constitute an optimal conglomerate that is yet to be unveiled for tooth regeneration. SDF1 is chemotactic and anti-apoptotic for bone marrow stem/progenitor cells and endothelial cells[55]. Our data show not only more homed cells, but also more vasculature upon combined SDF1 and BMP7 delivery. SDF1 binds to CXCR4, a chemokine receptor for endothelial cells and bone marrow stem/progenitor cells[55],[56]. Here, SDF1 likely has homed mesenchymal and endothelial stem/progenitor cells in alveolar bone into the porous tooth scaffolds in the rat jaw bone, and con- nective tissue progenitor cells in the dorsal subcutaneous tissue[65],[66],[67]. On the other hand, BMP7 likely is responsible for the ectopic mineralization in the dorsum and, importantly, newly formed bone in scaffold’s interface in tooth extraction socket. The present scaffold design represents the first anatomically dimensioned tooth scaffolds, and a variation from previous approaches in tooth regeneration by relying primarily on soft materials such as collagen gel, silk or PLGA[9],[28],[68]. PCL-HA composite offers mechanical stiffness that is suitable for load-bearing[69]. Among rapid prototyping methods, 3D bioprinting offers the advantage of precise control of pore size, porosity, permeability, stiffness and interconnectivity as well as anatomic dimensions[53],[68]. Clinically, the patient’s healthy, contra-lateral tooth form can be imaged by CT or MR, and then fed to a computer-aided design and a bioprinter to generate 3D scaffolds. Anatomically dimensioned scaffolds can either be patient-specific or of generic sizes, and made available as off-the-shelf im- plants in dental offices.
The present study, being the first of its kind for de novo formation of tooth-like tissues by cell homing, is not without limitations. All in vivo harvested samples were embedded in PMMA, because PCL-HA cannot be decalcified for paraffin embedding. PMMA embedding disallows immunoblotting by antibodies. Our ongoing work attempts to further characterize regenerated tissues by various imaging modalities. The regenerated mandibular incisor-like structure was mostly the root with a portion of subocclusal crown. We suggest that a rege- nerated tooth is biological primarily because of its root, rather than the crown that can be readily restored with a clinical crown anchorable to a biologically regenerated root[70].
Tooth regeneration: future directions The doctrine of cells, biomaterial scaffolds and signaling molecules has been the guiding principle in tissue engineering. Given the vast diversity of tissues that are being regenerated, it is difficult to conceive that one doctrine would govern all. In tooth regeneration, the doctrine of cells, biomaterial scaffolds and signaling molecules is considered below: Fig 3: stem cells and scaffold
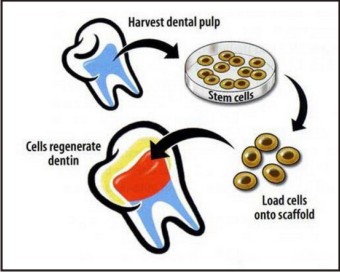 | Figure 3 : Stem Cells And Scaffold
 |
Cells
-
Embryonic tooth bud cells are not accessible as an autologous cell source for tooth regeneration in human.
-
Allogeneic (human) embryonic tooth bud cells are associated with ethic issues and limited availability
-
Xenogenic embryonic tooth bud cells may lead to dysmorphogenesis of regenerated teeth, even if it is applicable to humans.
Adult stem/progenitor cells from the third molar (wisdom tooth) or extracted teeth, per current practice, will need to be expanded ex vivo, manipulated and then transplanted into the patient, leading to unbearable cost, potential pathogen contamination and tumorigenesis of long-term manipulated cells. Cells are indeed required for tooth regeneration; however, cells do not necessarily need to be transplanted. Tooth regeneration by cell homing is an under-explored approach and deserves to be further studied[12],[13].
Scaffolds
-
Biomaterial scaffolds are likely indispensible for tooth regeneration. A tooth is a biological organ but also a structural material that withstand mechanical forces in mastication.
-
Ideal scaffolds for tooth regeneration should allow functionality of multiple cell types including odontoblasts, cementoblasts, pulp fibroblasts, vascular cells and/or neural endings, and potentially ameloblasts.
-
Ideal scaffolds for tooth regeneration must be clinically viable, i.e. easy to handle up to the point of a turn-key approach, can be readily sterilized and with a reasonably long shelf life.
-
Ideal scaffolds for tooth regeneration should be biocompatible, non-toxic and may need to undergo biologically safe degradation.
-
Either native or synthetic polymers, or a hybrid, are valid choices as scaffolding materials for tooth regeneration.
Signaling molecules
-
If biomaterial scaffolds are sufficient to recruit cells for tooth regeneration, signaling molecules are not needed.
-
In our proof of concept study, SDF1 and BMP7 are capable of elaborating mineralization and induce the formation of multiple periodontal tissues including the periodontal ligament and newly formed alveolar bone.
-
There is a need to determine the minimally needed signaling molecule(s) that is necessary for regeneration of tooth structures.
In summary, progress has been made to regenerate teeth with both cell transplantation and cell homing approaches (Table 2). One of the pivotal issues in tooth regeneration is to devise clinically translatable approa- ches that are not cost-prohibitive and can translate into therapies for patients who cannot afford or do not have access to dental implants. Costs for development of cell homing approaches for tooth regeneration are anti- cipated not as substantial as for tooth regeneration by cell transplantation. Molecular cues can be packaged and made available off-the-shelf in devices for tooth regeneration. In contrast, cell transplantation relies on costly procedures including procurement, ex vivo processing, potential cryopreservation, packaging, shipping, handling, and re-implantation into the patient. Thus, tooth regeneration by cell homing may provide tangible pathways towards clinical translation.
References
1. Poole D. Structural and chemical organization of teeth. New York: Academic Press, 1967.
2. Cuozzo FP, Sauther ML. Tooth loss, survival, and resource use in wild ring-tailed lemurs (Lemur catta): implications for inferring conspecific care in fossil hominids. J Hum Evol 2004; 46: 623–631.
3. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366: 1809–1820.
4. Sonoki K, Takata Y, Ansai T, et al. Number of teeth and serum lipid peroxide in 85-year-olds. Community Dent Health 2008; 25: 243–247.
5. Ostberg AL, Nyholm M, Gullberg B, Råstam L, Lindblad U. Tooth loss and obesity in a defined Swedish population. Scand J Public Health 2009; 37: 427–433.
6. Jung SH, Ryu JI, Jung DB. Association of total tooth loss with socio-behavioural health indicators in Korean elderly. J Oral Rehabil 2010; doi: 10.1111/j.1365-2842.2010.02178.x. [Epub ahead of print].
7. Polzer I, Schimmel M, Müller F, Biffar R. Edentulism as part of the general health problems of elderly adults. Int Dent J 2010; 60: 143–155.
8. Ferreira CF, Magini RS, Sharpe PT. Biological tooth replacement and repair. J Oral Rehabil 2007; 34: 933–939.
9. Modino SA, Sharpe PT. Tissue engineering of teeth using adult stem cells. Arch Oral Biol 2005; 50: 255–258.
10. Young CS, Abukawa H, Asrican R, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng 2005; 11: 1599–1610.
11. Mao JJ, Giannobile WV, Helms JA, et al. Craniofacial tissue engineering by stem cells. J Dent Res 2006; 85: 966–979.
12. Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res 2010; 89: 842–847.
13. Kim JY, Xin X, Moioli EK, et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A 2010; 16: 3023–3031.
14. Thesleff I, Järvinen E, Suomalainen M. Affecting tooth morphology and renewal by fine-tuning the signals mediating cell and tissue interactions. Novartis Found Symp 2007; 284: 142–163.
15. Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta 2009; 1790: 1592–1598.
16. Huang GT. Pulp and dentin tissue engineering and rege- neration: current progress. Regen Med 2009; 4: 697–707.
17. Zeichner-David M. Regeneration of periodontal tissues: cementogenesis revisited. Periodontol 2000 2006; 41: 196– 217.
18. Foster BL, Popowics TE, Fong HK, Somerman MJ. Advances in defining regulators of cementum development and perio- dontal regeneration. Curr Top Dev Biol 2007; 78: 47–126.
19. Cooke JW, Sarment DP, Whitesman LA, et al. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng 2006; 12: 1441–1450.
20. Lin NH, Gronthos S, Mark Bartold P. Stem cells and future periodontal regeneration. Periodontol 2000 2009; 51: 239– 251.
21. Pellegrini G, Seol YJ, Gruber R, Giannobile WV. Pre-clinical models for oral and periodontal reconstructive therapies. J Dent Res 2009; 88: 1065–1076.
22. Somerman M. Growth factors and periodontal engineering: where next? J Dent Res 2011; 90: 7–8.
23. Huang Z, Sargeant TD, Hulvat JF, et al. Bioactive nano- fibers instruct cells to proliferate and differentiate during enamel regeneration. J Bone Miner Res 2008; 23: 1995– 2006.
24. Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev 2008; 108: 4754– 4783.
25. Zhang J, Jiang D, Zhang J, Lin Q, Huang Z. Synthesis of dental enamel-like hydroxyapatite through solution mediated solid-state conversion. Langmuir 2010; 26: 2989–2994.
26. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920–926.
27. Nakao K, Morita R, Saji Y, et al. The development of a bioengineered organ germ method. Nat Methods 2007; 4: 227–230.
28. Ikeda E, Morita R, Nakao K, et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A 2009; 106: 13475–13480.
29. Mao JJ, Vunjak-Novakovic G, Mikos AG, Atala A. Trans- lational approaches in tissue engineering and regenerative medicine. Boston: Artech House, 2008.
30. Young CS, Terada S, Vacanti JP, et al. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res 2002; 81: 695–700.
31. Duailibi MT, Duailibi SE, Young CS, et al. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res 2004; 83: 523–528.
32. Young CS, Kim SW, Qin C, et al. Developmental analysis and computer modelling of bioengineered teeth. Arch Oral Biol 2005; 50: 259–265.
33. Duailibi SE, Duailibi MT, Zhang W, et al. Bioengineered dental tissues grown in the rat jaw. J Dent Res 2008; 87: 745–750.
34. Kuo TF, Huang AT, Chang HH, et al. Regeneration of dentin-pulp complex with cementum and periodontal liga- ment formation using dental bud cells in gelatin-chondroitin- hyaluronan tri-copolymer scaffold in swine. J Biomed Mater Res A 2008; 86: 1062–1068.
35. Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem- cell-based tissue engineering of murine teeth. J Dent Res 2004; 83: 518–522.
36. Mantesso A, Sharpe P. Dental stem cells for tooth regeneration and repair. Expert Opin Biol Ther 2009; 9: 1143–1154.
37. Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 2006; 1: e79.
38. Xu WP, Zhang W, Asrican R, et al. Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng Part A 2008; 14: 549–557.
39. Abukawa H, Zhang W, Young CS, et al. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J Oral Maxillofac Surg 2009; 67: 335– 347.
40. Ten Cate AR. Oral Histology. 5th Edition. St. Louis: Mosby, 1998.
41. Dammaschke T, Steven D, Kaup M, Ott KH. Long-term survival of root-canal-treated teeth: a retrospective study over 10 years. J Endod 2003; 29: 638–643.
42. Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol 2002; 18: 134–137.
43. Caplan DJ, Cai J, Yin G, White BA. Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent 2005; 65: 90–96.
44. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod 2007; 33: 377–390.
45. Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 2007; 13: 151–157.
46. Hargreaves KM, Giesler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J Endod 2008; 34: S51–S56.
47. Galler KM, Cavender A, Yuwono V, et al. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng Part A 2008; 14: 2051–2058.
48. Gotlieb EL, Murray PE, Namerow KN, Kuttler S, Garcia- Godoy F. An ultrastructural investigation of tissue-engi- neered pulp constructs implanted within endodontically treated teeth. J Am Dent Assoc 2008; 139: 457–465.
49. Prescott RS, Alsanea R, Fayad MI, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after sub- cutaneous transplantation in mice. J Endod 2008; 34: 421– 426.
50. Huang GT, Yamaza T, Shea LD, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 2010; 16: 605–615.
51. Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008; 34 :962–969.
52. Mao JJ, Stosich MS, Moioli EK, et al. Facial reconstruction by biosurgery: cell transplantation versus cell homing. Tissue Eng Part B Rev 2010; 16: 257–262.
53. Lee CH, Marion NW, Hollister S, Mao JJ. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng Part A 2009; 15: 3923–3930.
54. Stosich MS, Moioli EK, Wu JK, et al. Bioengineering strategies to generate vascularized soft tissue grafts with sustained shape. Methods 2009; 47: 116–121.
55. Belema-Bedada F, Uchida S, Martire A, Kostin S, Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell 2008; 2: 566–575.
56. Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 2009; 60: 813– 823.
57. Goldberg M, Six N, Decup F, et al. Application of bioactive molecules in pulp-capping situations. Adv Dent Res 2001; 15: 91–95.
58. Rutherford RB. BMP-7 gene transfer to inflamed ferret dental pulps. Eur J Oral Sci 2001; 109: 422–424.
59. White AP, Vaccaro AR, Hall JA, et al. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop 2007; 31: 735–741.
60. Nait Lechguer A, Kuchler-Bopp S, Hu B, Haïkel Y, Lesot H. Vascularization of engineered teeth. J Dent Res 2008; 87: 1138–1143.
61. Hahn GV, Cohen RB, Wozney JM, et al. A bone morphogenetic protein subfamily: chromosomal localization of human genes for BMP5, BMP6, and BMP7. Genomics 1992; 14: 759–762.
62. Itoh F, Asao H, Sugamura K, et al. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J 2001; 20: 4132–4142.
63. Lee CH, Cook JL, Mendelson A, et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 2010; 376: 440–448.
64. Yelick PC, Vacanti JP. Bioengineered teeth from tooth bud cells. Dent Clin North Am 2006; 50: 191–203.
65. Alhadlaq A, Mao JJ. Mesenchymal stem cells:isolation and therapeutics. Stem Cells Dev 2004; 13: 436–448.
66. Steinhardt Y, Aslan H, Regev E, et al. Maxillofacial-derived stem cells regenerate critical mandibular bone defect. Tissue Eng Part A 2008; 14: 1763–1773.
67. Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci 2009; 1176: 118–123.
68. Woodfield TB, Van Blitterswijk CA, De Wijn J, et al. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-e ngineered cartilage constructs. Tissue Eng 2005; 11: 1297– 1311.
69. Application of dedifferentiated fat cells for periodontal tissue regeneration. Sugawara A, Sato S.Hum Cell. 2013 Sep 26.
70. rhBMP-2/ACS Grafts Versus Autogenous Cancellous Marrow Grafts in Large Vertical Defects of the Maxilla: An Unsponsored Randomized Open-Label Clinical Trial.Marx RE, Armentano L, Olavarria A, Samaniego J.Int J Oral Maxillofac Implants. 2013 Sep-Oct;28(5):e243-51.
|