Introduction
Anatomic aberrations are seen often in the human dentition. The maxillary incisor region of the permanent dentition where these anatomical aberrations are commonly seen is considered an area of embryonic hazard. Aberrations affecting the external and internal morphology can at times be the cause of complex pathological conditions involving the pulpal and periodontal tissues and can pose a challenge to the clinician for diagnosis and clinical management. One such anatomical aberration is a developmental groove involving the crown and extending a variable distance onto the root. The palatal surface of the maxillary lateral incisor and labial surface of the maxillary central incisor are most commonly involved. Such developmental grooves affecting maxillary incisors are termed palatoradicular grooves. This anomaly also has been termed radicular anomaly, palatogingival groove, distolingual groove and radicular lingual groove (Prichard 1965, Lee et al. 1968, Simon et al. 1971, Everett & Kramer 1972).[1],[2],[3],[4] This anomaly can pose dilemmas for diagnosis and clinical management. A majority of palatoradicular grooves (93.8%) affect maxillary lateral incisors (Everett & Kramer 1972), and may result from an infolding of the enamel organ and the epithelial rootsheath of Hertwig (Lee et al. 1968).[2],[4] Some have suggested that the anomaly results from an attempt to form another root (Lee et al. 1968, Peikoff & Trot 1977)[5]. Kovacs (1971) called this anomaly ‘syndesmocorono-radicular tooth’[6]. These grooves act as a nidus for plaque accumulation which destroys the sulcular epithelium and later deeper parts of the periodontium, finally resulting in the formation of a severe localized periodontal lesion since proper cleaning of that site is difficult, if not impossible, for the patient. These grooves may also lead to combined endodontic-periodontal lesions, since there might be a communication between the pulp canal system and the periodontium through accessory canals. The prognosis of teeth affected by this anomaly depends upon the depth and extension of the groove. Shallow grooves may be corrected by odontoplasty in conjunction with periodontal treatment. However, when the groove is more advanced, treatment of the teeth is almost always doomed to failure either because of pulpal or periodontal breakdown. A case is presented of a maxillary lateral incisor with a shallow palatoradicular groove extending up to the root apex with severe periodontal destruction. The tooth was successfully managed by restorative and surgical periodontal therapy. The rationale behind treatment modalities is discussed. With a prevalence of 2–5% the PRGs are one of the rare anomalies of tooth development[7],[8]. Also it has been found that most of the grooves exhibit a length of more than 5 mm. The origin of PRG has been explained as a result of the alteration in the growth of inner enamel epithelium and Hertwig’s epithelial root sheath (HERS). Along with having numerous similarities to dens invaginatus, it has also been considered as an aborted attempt for the formation of an additional root[9]. Some authors have also suggested a genetic preponderance of this groove[10]. For the sake of understanding, PRGs have been classified as simple and complex[9]. While simple groove results from partial infolding of HERS, the complex one is characterized by direct communication with pulp. The most complex form of groove may even separate an accessory root from the main root trunk[9]. Based on location they are classified as distal, mesial and central pattern. The distal pattern is most common and has been witnessed in approximately 70% of cases[11].
Case Report
A 22-years-old female patient reported to the Department of conservative dentistry & endodontics of H.P.Govt. Dental College & Hospital, shimla, with the chief complaint of persistent dull pain and severe sensitivity in the upper front teeth since few months. On clinical examination it was found that all the teeth in the maxillary right quadrant were intact, caries-free, with mobility within physiological limit and non-tender on percussion. Patient’s general medical history was taken and was found to be non-relevant. The oral hygiene status of the patient was also satisfactory. A small notch was evident on the palatal aspect of the tooth crown 22 (Fig. 1).
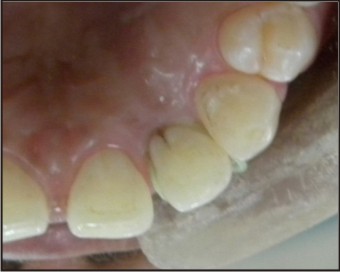 | Fig 1 : Preoperative Photograph Tooth No. 22
 |
Careful periodontal examination of the patient revealed an 8 mm pocket on the mid-palatal aspect of maxillary lateral incisor along with some amount of purulent discharge the source of which was confirmed by by using gutta percha tracing on IOPA x-ray (Fig. 2).
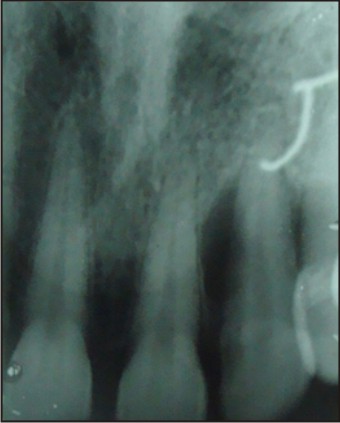 | Fig 2 : Preoperative Iopa Tooth No. 22
 |
The tooth was investigated for vitality and was found to be vital on thermal and electric pulp testing. The radiographic and clinical findings revealed that the tooth was not having any pulpal involvement. A combined restorative-periodontic treatment plan was then formulated and was proceeded for. In the first phase of treatment it was decided to remove the cause for the pus discharge and promote periodontal regeneration. In consultaion with a periodontist a flap was raised to debride the periapical area and promote bone regeneration around the tooth. After careful debridement the flap was repositioned and sutured using 3-0 silk suture (Fig. 3, 4, 5, 6, 7).
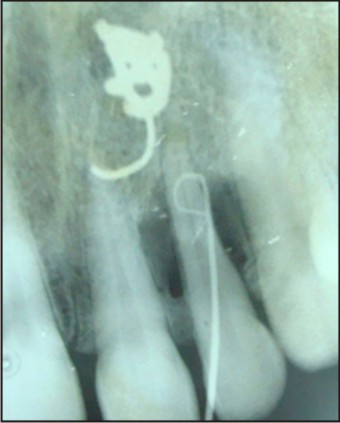 | Fig 3 : Iopa Showing Gutta Percha In Defect
 |
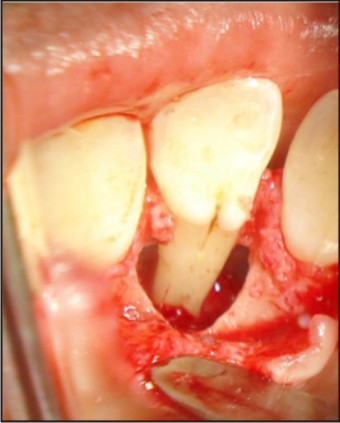 | Fig 4 : Palatoradicular Groove
 |
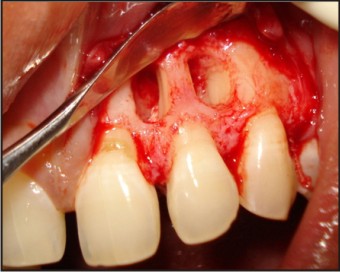 | Fig 5 : Unusual Bony Defects On Labial Side
 |
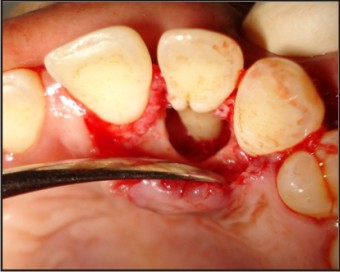 | Fig 6 : Saucerization Of Groove
 |
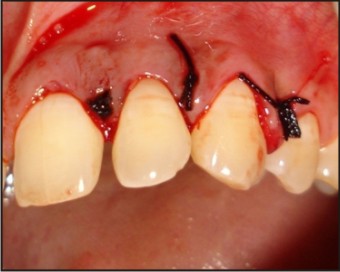 | Fig 7 : Flap Approximated And Sutures Given
 |
The patient was given routine postoperative instructions along with Ibuprofen 400 mg to be taken twice daily for 3 days. After 1week healing was uneventful and the sutures were removed. The patient was advised to maintain oral hygiene and report after 1 week for coronal groove restoration using flowable composite (Fig. 8, 9, 10, 11, 12).
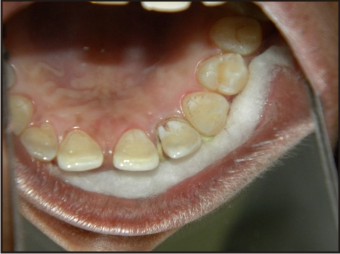 | Fig 8 : Coronal Part Of Groove After 2 Weeks
 |
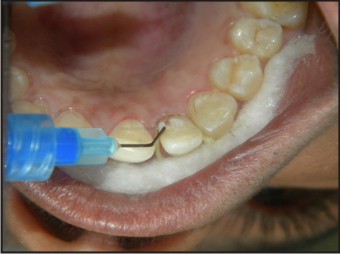 | Fig 9 : Application Of Etchant
 |
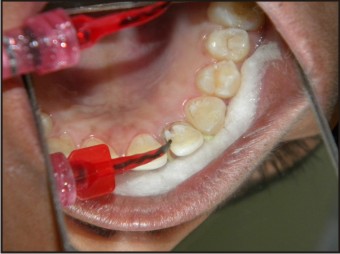 | Fig 10 : Application Of Bonding Agent
 |
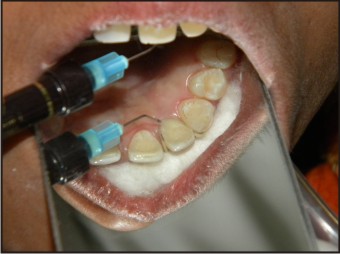 | Fig 11 : Application Of Flowable Composite
 |
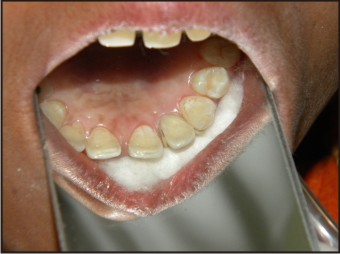 | Fig 12 : After Coronal Restoration
 |
After 6 months of the treatment patient was asymptomatic and satisfied with the treatment outcome (Fig. 13, 14).
 | Fig 13 : Follow Up After 6 Months
 |
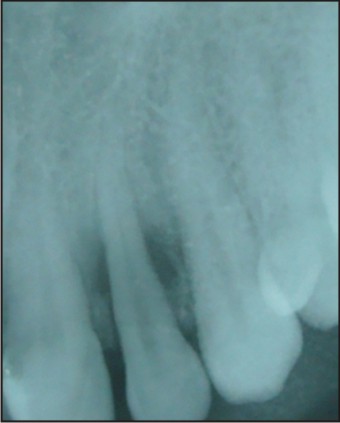 | Fig 14 : Follow Up Iopa Tooth No.22
 |
Discussion
Palatoradicular groove is a rare developmental anomaly with a prevalence of 2.8–8.5% (Everett & Kramer 1972, Withers et al. 1981)[4],[12]. This range represents different occurrences in different populations and sub-populations. Also it has been found that most of the grooves exhibit a length of more than 5 mm. The origin of PRG has been explained as a result of the alteration in the growth of inner enamel epithelium and Hertwig’s epithelial root sheath (HERS). Along with having numerous similarities to dens invaginatus, it has also been considered as an aborted attempt for the formation of an additional root[9]. Some authors have also suggested a genetic preponderance of this groove[10]. For the sake of understanding, PRGs have been classified as simple and complex[9]. While simple groove results from partial infolding of HERS, the complex one is characterized by direct communication with pulp. The most complex form of groove may even separate an accessory root from the main root trunk. Based on location they are classified as distal, mesial and central pattern. The distal pattern is most common and has been witnessed in approximately 70% of cases[11]. The occurrence of grooves extending from Cingulum to apex, as in this case, appears to be extremely rare (Everett & Kramer 1972)[4]. Patient with a PRG is not always symptomatic. In many cases it has been reported that delayed or improper treatment of a pathological groove was the consequence of an improper diagnosis. As suggested by Robinson, early diagnosis of PRG requiring periodontal treatment would simplify the treatment and improve the prognosis[13]. The treatment options for PRG may vary depending upon the depth and extent of the groove. Shallow one may be eliminated by saucerization which involves grinding or flattening of root surface[14]. But the use of this method in deep grooves is questionable because of the excessive wear and tear of dentin which is obvious. In most of the cases PRG is complicated with pulpal involvement and requires an endodontic treatment trends in periodontal surgical therapy of PRG include the use of alloplastic graft materials and mechanical barrier membrane to facilitate bone regeneration. In this case, any such method was not chosen as the use of materials like barrier membrane and bone graft as very limited studies have shown tangible effects of these materials during periodontal healing, also the patient was reluctant to pay for the additional cost of treatment using bone grafts[15][16][17][18]. Instead, emphasis was given on removal of inflammation and making the environment more conducive for regeneration. The 3-mm non-bleeding sulcus at 6 months’ recall proved the clinical success of case which was mainly achieved due to thorough removal of inflammatory agents and obliteration of groove.
Summary
Palatogingival grooves are uncommon in maxillary lateral incisors, but when present may contribute to the pathogenesis of periodontal and endodontic lesions. Successful outcome of PRG follows accurate diagnosis and elimination of irritants and contributing factors. Correct evaluation of clinical signs and meticulous examination for revealing the extent of the lesion is of utmost importance to avoid an incorrect course of treatment which may ultimately lead to poor prognosis and even loss of the tooth. Most of the diagnosed cases require an interdisciplinary approach for successful management of this pathology.
References:
1. Prichard JS (1965) Advanced Periodontal Therapy. Philadelphia, PA: WB Saunders Co.
2. Lee KW, Lee EC, Poon KY (1968) Palato-gingival grooves in maxillary incisors. British Dental Journal 124, 14–8.
3. Simon JH, Glick DH, Frank AL (1971) Predictable endodontic and periodontal failures as a result of radicular anomalies. Oral Surgery, Oral Medicine, Oral Pathology 31, 823–6.
4. Everett FG, Kramer GM (1972) The distolingual groove in the maxillary lateral incisor: a periodontal hazard. Journal of Periodontology 43, 352–61.
5. Peikoff MD, Trot JR (1977) An endodontic failure caused by an unusual anatomical anomaly. Journal of Endodontics 3, 356–9.
6. Kovacs I (1971) A systemic description of dental roots. In: Dhalberg AA, ed. Dental Morphology and Evolution. Chicago, IL: University of Chicago Press, pp. 223–5.
7. Hou Gl, Tsai CC. Relationship between palatoradicular groove and localised periodontitis. J Clin Periodontol 1993;20:678–82.
8. Schwartz SA, Koch MA, Deas DE, Powell CA. Combined endodontic-periodontic treatment of a palatal groove: A case report. J Endod 2006; 32:573–78.
9. Goon WW, Carpenter WM, Brace NM, Ahlfeld RJ. Complex facial radicular groove in a maxillary lateral incisor. J Endod 1991;17: 244–8.
10. Ennes JP, Lara VS. Comparative morphological analysis of the root developmental groove with the palato-gingival groove. Oral Dis 2004; 10:378–82.
11. Vanessa SL, Alberto C, Robert SB. Macroscopic and microscopic analysis of the palato-gingival groove. J Endodontics 2000;26:345–50.
12. Withers JA, Brunsvold MA, Killoy WJ, Rahe AJ. The relationship of palato-gingival grooves to localized periodontal disease. J Periodontal 1981;52:41–44.
13. Robinson SF, Cooley RL. Palatogingival groove lesion: recognition and treatment. Gen Dent 1988;36:340–2.
14. Schafer E, Cankay R, Ott K. Malformations in maxillary incisors: case report of radicular palatal groove. Endod Dent Traumatol 2000; 16:132–7.
15. Wang HL, Macneil RL. Guided tissue regeneration absorbable barriers. Dental Clinics of North America 1998;42:503–41.
16. Wang HL, Yuan K, Syed S, et al. Adherence of oral microorganisms to guided tissue regeneration membranes—an in vitro study. J Periodontol 1994; 65:211–8.
17. Ricci G, Rasperini G, Silvestri M. In vitro permeability evaluation and colononization of membrane for periodontal regeneration by Porpyyromonas gingivalis. J Periodontol 1996;67:490–6.
18. Buser D, Dula K, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes—a clinical study with 40 par- tially edentulous patients. J Oral Maxillofacial Surg 1996;54:420–32.
|