Introduction
Endodontic therapy mainly aims at elimination of microorganisms from the root canal and prevents reinfecton. Since some of these micro organisms can remain in the root canal and dentinal tubules even after root canal instrumentation[1], further disinfection with the use of antimicrobial dressings is generally recommended[2]. Such medicaments must have greatest antimicrobial efficacy against various bacterial species, without causing irritation to periapical tissue. Calcium hydroxide suspension in paste form has been shown to meet these demands to high degree[3]. Therefore Ca(OH)2 is considered the intracanal medicament of choice.
Since ancient times, plants have been good source of medicines as they serve as a reservoir of chemical agents with therapeutic properties. However, it is only off late, that researchers have employed scientific methods to prove the efficacy and provide a better understanding of the mechanisms of action[4]. At present, it is estimated that about 80% of the world population relies on botanical preparations as medicines to meet their health needs.
The evergreen tree, Neem, one of the most versatile medicinal plant, extensively used in traditional medicine, has been found to exert antibacterial, antimalarial, contraceptive and antiulcerative properties[5],[6],[7]. Ginger belonging to Zingiberaceae family has also been used as medicine from Vedic period and is called ‘Maha aushadi’, which means great medicine exhibiting strong antibacterial and antifungal properties[8].
Most endodontic infections are polymicrobial, with predominance of strict anaerobes, some facultative anaerobes and rarely aerobes[9]. Entercoccus faecalis, which is a gram positive facultative anaerobe, has been frequently isolated from failed root canal therapies. This has been found to be due to its ability to survive in protected environments like biofilms, inside dentinal tubules where they are less likely to come in contact with antimicrobial irrigants. Moreover, it grows in high salt concentrations, wide temperature range and tolerates a broad pH range and starves until an adequate nutritional supply becomes available. When the conditions are favorable they multiply and repopulate in the canal system, thereby causing reinfection and treatment failure[10],[11],[12],[13],[14]. Because of this E.faecalis has been used in numerous studies to evaluate the antibacterial properties of disinfecting agents.
Antimicrobial effects of natural plant extracts on endodontic pathogens have generally not been evaluated and reported. So the purpose of this study is to evaluate the antibacterial effect of Neem, Ginger and its combination with calcium hydroxide using a Modified Direct Contact Test against E.faecalis.
Materials And Methods:
Preparation of extract
The fresh plant materials were obtained from the local market. Fresh ginger was cleaned, descaled, and washed in sterile distilled water. Neem leaves were washed in sterilized distilled water and macerated with 50ml of absolute ethanol. About 100mg of cleaned ginger and neem were crushed with mortar and pestle. Each extracts were sieved through a fine mesh cloth and sterilized using a membrane filter (0.45 micron sterile filter). This extract was considered as the 100% conc. of the extract. The 60% Ca(OH)2 pH (12.3) (Deepashree products, Ratnagiri, India.) was made up by mixing 60mg Ca(OH)2 powder with 100ul distilled water[15]. A combination of 60% Ca(OH)2 and plant extract (1:1 v/v) was also used in this study.
Microorganism
E.fecalis strain(ATCC 29212) were taken from frozen samples grown overnight in air at 370C on TSA plates (BBL Microbiology Systems, Cockeysville, MD,USA) for 24h. After checking for purity, E.fecalis was suspended in sterile water and adjusted to density of 3x108 CFU/ml by using a microplate reader model at 405nm.
Modified DCT;
A 96 well micro titer plate was held vertically and an area of fixed size on the side wall of the wells was coated with an equal amount (100ul) of each extract with an applicator tip.
A 10ul of bacterial suspension (3x108 cfu/ml. which contained 3x106 bacteria) was carefully placed on the surface of each extract. Bacterial suspensions placed on the wall of uncoated wells were used as control. After incubation in 100% humidity at 370C for 24hrs, 240ul of Trypticase Soya Broth was added to each well. After gently mixing with pipette for 1min, the bacterial suspension from each well was transferred and serially diluted in TSB. The survival of bacteria was assessed by culturing aliquots of 20ul onto TSA plate after 10-fold serial dilutions. After incubation for 24 hrs at 370C, colonies on the plates were counted and the cfu/ml was calculated. All experiments were performed in triplicate.
Results
The result of the antibacterial effects of medicinal plants from modified DCT is presented in Table 1. The growth of E.fecalis was measured as absorbance and analyzed with t-test. Higher mean bacterial count is recorded in Ginger compared to Neem and the difference between them is found to be statistically significant (P<0.001) as shown in Graph 1.
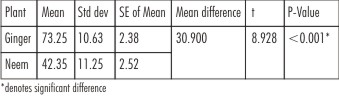 | Table 1 : Growth Of E.Faecalis (Level Of Significance: A=0.05)
 |
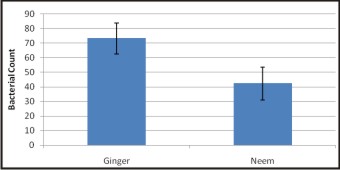 | Graph 1 : Mean Bacterial Count Of Ginger And Neem
 |
The results of antibacterial effects of Ca(OH)2 and combination of Ca(OH)2 /Neem and Ca(OH)2 /ginger is analyzed by Anova followed by Bonferroni test for multiple comparisons as presented in Table 2 and 3. Higher mean bacterial count is recorded in Ca(OH)2 followed by Ca(OH)2 /ginger and Ca(OH)2 /neem and the difference between them is found to be statistically significant (p<0.001) as shown in Graph 2.
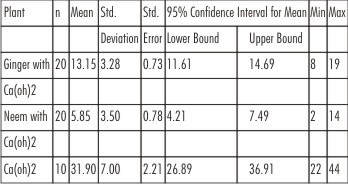 | Table 2 : Anova Test To Compare Within The Groups
 |
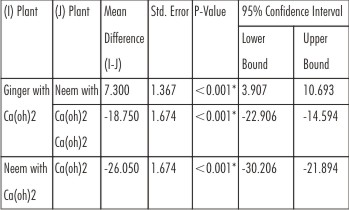 | Table 3 : Comparision Between Groups Using Bonferroni Method
 |
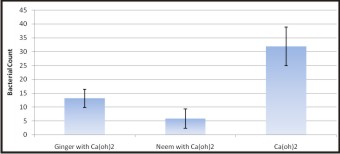 | Graph 2 : Mean Bacterial Count Of Ginger, Neem And Calcium Hydroxide
 |
Discussion
Recent years have seen an increased enthusiasm in treating various diseases with natural products. The major advantages of using herbal alternatives are easy availability, cost effectiveness, increased shelf life, low toxicity and lack of microbial resistance. Thus, spices which are normal ingredients of our routine food preparations can provide protection to a certain extent against our natural enemies like bacterial pathogens.
Several plants serve as a source of therapeutic agents containing antibiotics and other medicinal compounds[16],[17]. The active substances isolated from these plants have shown to posses potent antimicrobial, analgesic and anti-inflammatory properties[18],[19],[20]. Hence, an attempt has been made to evaluate antibacterial activity of Neem and Ginger and its combination with calcium hydroxide against E.fecalis.
E.faecalis survives in the root canal as a single organism and multiplies, causing infection. The cells of E.faecalis remain viable and have the capability to invade the dentinal tubules and adhere to the collagen in the presence of human serum, thus acting as a pathogen in failed endodontically treated teeth[21]. Thus, this microorganism was used in this study.
The agar diffusion test used to be the most commonly applied method to assess the antimicrobial activity of different materials. However, because of their limitations, it is no longer recommended to be used for this purpose[22],[23]. A Direct Contact Test, which circumvents many of the problems of ADT, was first introduced by Weiss et al[14] for the evaluation of the antimicrobial effect of endodontic sealers and root-end filling materials. This test is a quantitative and reproducible assay that allows testing of various materials and can be used in standardized settings. The Modified Direct Contact test used in this study was followed according to Huizang et al[24]. It was modified in such a way that plating was done immediately after each time of contact. This modification makes it possible to measure the bactericidal effect instead of bacteriostatic effect of the materials. It is both qualitative and quantitative reproducible assay. It also makes it possible to directly calculate the exact numbers of surviving bacteria after each contact time.
Sixty percent concentration of Ca(OH)2 was selected because it was recommended by Blanset et al[25] for use as an endodontic inter appointment medicament. They indicated that formulation 50 to 60% aqueous Ca(OH)2 resulted in the greatest overall bacterial inhibition of endodontic pathogens including E.fecalis.
The results of the present study showed that Neem alone and its mixture with Ca(OH)2 had better bactericidal effect than Ginger and ginger/ Ca(OH)2 , whilst Ca(OH)2 alone had only a marginal amount of antibacterial effect against E.fecalis which is in agreement with some studies[26],[27]. This bacterium appears to be highly resistant to Ca(OH)2 at a pH of 11.1, but unable to survive at a PH higher than 11.5. In radicular dentine, due to its buffer effect, the alkanity of Ca(OH)2 may only reach a pH of 10.3 after intracanal dressing[28]. This could be one of the factors contributing to resistance of E.fecalis to Ca(OH)2.
In fresh ginger rhizome, the gingerols were identified as the major active component to exert antibacterial activity [29]. Neem exhibited better bactericidial effect mainly due to presence of particular chemical substances that have a definite physiological action on the living system. The most important of these are alkaloids, glycosides, saponins, flavonoids, steroids, anthraquinone and tannic acid[30]. Of all these flavnoids act as antioxidants which provide protection against free radicals that damage cells and tissues. Moreover, its antiadherence activity alters bacterial adhesion and ability of organism to colonize[31]. Also Botelho et al[32] and Behl et al in their experiments and trials concluded that Neem is highly efficacious in the treatment of periodontal disease thus exhibiting its biocompatibility with human periodontal ligament fibroblasts [33]. The possible reason for the antimicrobial action of calcium hydroxide with neem may be due to additive or synergistic effects.
Conclusion
Under the limitations of this study, mixture of Ca(OH)2 and Neem had better antibacterial effect than Ca(OH)2 alone and Ginger. As the global scenario is now changing towards the use of non-toxic plant products that have traditional use, further studies are required for the clinical application of the results of this in-vitro study before neem can conclusively recommended as an intracanal medicament. Search for new antimicrobials is very important in recent times, considering the escalating levels of antibiotic resistance among pathogenic bacteria.
References
1. Barnett F, Trope M, Khoja M, Tronstad L. Bacteriologic Status of the root canal after sonic, ultrasonic and hand instrumentation. Endodontics and Dental Traumatology 1985;1:228-31.
2. Sjogren U, Figdor D, Spanberg L, Sundquist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. International Endodontic journal 1991;24:119-25.
3. Siqueria JF Jr, Lopes HP. Mechanism of antimicrobial activity of calcium hydroxide: a critical review. International Endodontic journal 1999;32,361-9.
4. Graf J. Herbal anti-inflammatory agents for skin disease. Skin Therapy Letter 2000;5:3-5.
5. Siddiqui S, F, aizi s, Siddiqui B. S and Ghiasuddin. Constituents of Azadirachta indica: isolation and structure elucidation of a new antibacterial tetranortriterpenoid, mahmoodin and a new protolimonoid, naheedin. J Nat Prod 1992;55:303-310.
6. Jones I W, Denholm A A, Ley S V, Lovell H, Wood A and Sinden R E. Sexual development of malarial parasites is inhibited in vitro by neem extract azadirachtin and its semi-synthetic analogues. FEMS Microbial Letter 1994;120:267-273.
7. Garg G P, Nigam S K and Ogle C W. The gastric antiulcer effects of the leaves of the neem tree. Planta Med 1993;59:215-217.
8. ICMR Bulletin. Ginger: Its role in xenobiotic metabolism 2003;33:6
9. Peciuliene V, Balciuniene I, Eriksen H M, Haapasalo M. Isolation of Enterococcus fecalis in previously root-filled canals in Lithuanian population. J Endod 2000;26:593-5.
10. Slutzky-Goldberg I, Slutzky H, Solomonov M, Moshonov J, Weiss EI, Matalon S. Antibacterial properties of four endodontic sealers, J Endod 2008;34:735-8.
11. Bodrumlu E, Semiz M. Antibacterial activity of a new endodontic sealers against Enterococcus faecalis. J Can Dent Assoc 2006;72:637.
12. Siqueria JF Jr, Favieri A, Gahyva SM, Moraes SR, Lima KC, Lopes HP. Antimicrobial activity and flow rate of newer and established root canal sealers. J Endod 2000;26:274-7.
13. Heling I, Chandler NP. The antimicrobial effect within dentinal tubules of four root canal sealers. J Endod 1996;22:257-9.
14. Weiss EI, Shalhav M, Fuss Z. Assessment of antibacterial activity of endodontic sealers by a direct contact test. Endod Dent Traumatol 1996;12:179-84.
15. Badr AE,Omar N and Badria FA. A laboratory evaluation of the antibacterial and cytotoxic effect of liquorice when used as root canal medicament. International Endodontic journal 2011;44;51-58.
16. Almas K and Al-lafi TR. The natural toothbrush. World Health Forum 1995;16:206-10.
17. Al-lafi T and Ababneh H. The effect of extract of the miswak (chewing sticks) used in Jordan and The Middle East on oral bacteria. Int Dent J 1995;45:218-22.
18. Wu-Yuan CD, Green L, Birch WX. In vitro screening of Chinese medicinal toothpastes: Their effects on growth and plkaque formation of mutans streptococci. Caries Res 1990;24:198-202.
19. Wolinsky LE and Sote EO. Isolation of natural plaque-inhibiting substances from Nigerian chewing sticks. Caries Res 1984;18:216-25.
20. Akpata ES and Akinrimisi EO. Antibacterial activity of extracts from some African chewing sticks. Oral Surg Oral Med Oral Pathol 1977;44:717-28.
21. R.M Love. Enterococcus fecalis- a mechanism for its role in endodontic failure. Int Endod J 2001;34:339-405.
22. Editorial Board of Jouranal of the Endodontics. Wanted :a baseof evidence. J Endod 2007;33:1401-2.
23. Haapasalo M, Qian W. Iriigants and intracanal medicaments. In Ingle JI, Bakland LK, Baumgartner JC, eds. Ingle’s endodontics. 6th ed. Hamilton, ON, Canada:BC Decker Inc:2008:992-1011.
24. Huizhang, Ya Shen N, Dorin Ruse and Markus Haapasalo. Antibacterial activity of endodontic sealers by Modified Direct Contact test against Enterococcus fecalis. J Endod 2009;35:1051-55.
25. Blanset ML, Tordik PA, Goodell GG. An agar diffusion comparison of the antimicrobial effect of calcium hydroxide at five different concentrations with three different vehicles. J Endod 2008;34,1246-8.
26. Safavi KE, Spangberg LS, Langeland K. Root canal dentinal tubule disinfection. J Endod 1990;16,207-10.
27. Peters LB, Van Winkelhoff AJ, Buis TF, Wesselink PR. Effects of instrumentation, irrigation and dressing with calcium hydroxide on infection in pulpless teeth with periapical bone lesions. . International Endodontic journal 2002; 35, 13-21.
28. Minana M, Carner DL Jr, Walker WA III. PH changes at the surface of root dentin after intracanal dressing with calcium oxide and calcium hydroxide. J Endod 2001; 27,43-45.
29. Tyler E,Brady LR and Robbers JE. Pharmacognsy (8th edition) Lea and Febiger, Philadelphia p156, 1981.
30. Faiza Aslam, Khalil-ur Rehman, Muhammad Asghar and Muhammad Sarwan. Antibacterial activity of various phytoconstituents of Neem. Pak J Agri Sci 2009;46(3):209-213.
31. S. Polaquini T, Svidzinski C, Kemmelmeier A, Gasparetto. Effects of aqueous extract from Neem on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Archives of Oral Biol 2006, 51(6):482-90.
32. Bothelho M, Araujo Dos Santos, Martins J, Carvalho C, Paz M, Azenhac et al. Efficancy of a mouthrinse based on leaves of Neem in the treatment of patients with chronic gingivitis. J Medicinal Plants Research 2008;2(11):341-46.
33. Behl H, Sidhu O, Kumar V, Singh D, Saimbi C. Efficacy of neem active metabolites for prevention of dental plaque and gingivitis. Neem Foundation 2002.
|