Introduction
Calcium hydroxide was introduced to dentistry by Hermann at the beginning of the 20th century, and since then it has been widely used in dentistry. It is a strong alkaline substance with a pH of approximately 12.5 and has various biologic properties that promoted its use in several clinical situations. Its dental use relates chiefly to its antibacterial properties and the ability to induce repair and to stimulate hard-tissue formation. The main benefit of calcium hydroxide as an intracanal medicament lies in its bactericidal effect conferred by its high pH, as many endodontic microorganisms are unable to survive in the highly alkaline environment provided by calcium hydroxide[1],[2].
History
In 1920, B.W. Hermann introduced calcium hydroxide for root canal fillings. Between 1928 and 1930, he studied the reaction of vital pulp tissue to calcium hydroxide to prove that it was a biocompatible material. Since then calcium hydroxide has been recommended by several authors for direct pulp capping. But it took until the middle of 20th century until it was regarded as the standard of care[3].
Zander and Glass in 1949 described the usefulness of calcium hydroxide in dentistry and endodontics. Zander exploited its usefulness as a pulp capping and pulpotomy agent and it later became widely used to help induce apexogenesis and apexification. Calcium hydroxide has become the medicament of choice where interim canal dressing is necessary. The works of Bystrom, Cvek and Tronstad have shown that calcium hydroxide was a canal dressing that maintained its antibacterial effect when sealed in the canal[4].
Properties
Calcium hydroxide, also known as calcium hydrate, lime hydrate, caustic lime or slaked lime is a soft white crystalline powder with alkaline, slightly bitter taste.
It is radiolucent and various materials like barium sulphate, diatrizoate meglumine or iothalamate meglumine are used to induce radiopacity.
pH of water solution at 250C is 12.4.
Its specific gravity is 2.34.
It is very slightly soluble in water, soluble in glycerin, acids and syrup.
Calcium hydroxide is produced from calcium oxide by hydrolysis.
CaO + H2O Ca(OH)2 + H – 62.7 KJ( evolved heat)
The natural impurities that may be present in calcium hydroxide are calcium carbonate, magnesium salts and Iron.
Mechanism of action[5],[6],[7],[8]
Mechanism of action of hydroxyl ions on bacteria:
Calcium hydroxide is an antibacterial agent due to its elevated pH. pH influences the specific activity of the proteins of the membrane, with a combination with specific chemical groups and can lead to alterations in the ionization state of organic components.
It denatures bacterial endotoxin.
Mechanism of action on tissues
Induces dentinogenesis. Elevated pH of calcium hydroxide activates alkaline phosphatase from the tissue. This is a hydrolytic enzyme and liberates phosphate from esters of phosphates. This phosphate ion once free will react with the calcium ion from the blood stream to form a precipitate of calcium phosphate in the organic matrix. This precipitate is the molecular unit of hydroxy apatite. Calcium hydroxide when in direct contact with adjacent tissue gives origin to zone of necrosis through rupture of glycoproteins resulting in protein degeneration with 7-10 days. Using a different methodology, electronic microscopy and microanalysis of dispersion of X-ray have confirmed this action.
Promotes healing of periapical tissue and hard tissue formation.
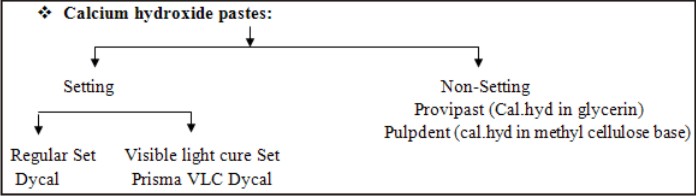 | Formulations/ Preparations of Calcium hydroxide
 |
Dry Powder:
Dry powder of calcium hydroxide is available which can be used as such or can be mixed with distilled water, normal saline, ringer lactate , anesthetic solution to form a paste.
Root Canal Sealers:
Calcibiotic Root Canal Sealer paste( CRCS paste) contains ZnOxide Eugenol and Cal.Hyd (P & L)
Sealapex contains cal.hyd in a polymerizing resin (two paste system)
Liquids
Supplied in bottles. Resinous solutions of cal.hyd used as liners.
Clinical Applications:
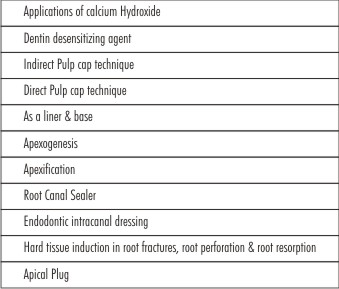
Calcium Hydroxide has gained many applications in restorative dentistry since its introduction by Hermann.It includes(Table I).
 | Table 1
 |
Dentin Desensitizing Agent:
Calcium hydroxide has been advocated for the relief of hypersensitive root dentine.
The proposed mechanisms for reducing dentine permeability include: (a) physical blockage of the tubule orifices, (b) production of precipitates or mineralizations, and (c) stimulation of secondary dentine[9].
Indirect Pulp Cap Technique:
One of the most important step in cavity preparation is removal of carious dentin but in some instances where removal of caries is expected to expose pulp it is advisable to use
indirect pulp cap technique. In this technique, the carious affected dentin nearest to pulp is left and calcium hydroxide is placed over it and the tooth is restored and kept under observation. Therefore the prime role for the placement of calcium hydroxide onto this deep softened dentine is to render it sterile through the antibacterial activity. Secondly, calcium hydroxide induces odontoblasts to produce reparative dentin below the existing dentin.The rate of reparative dentin formation on an average is 1.4 micrometer per day in human teeth[1].
Direct Pulp Cap Technique:
In direct pulp cap technique, the calcium hydroxide is directly applied to the exposed pulp which aims at formation of a calcific barrier to repair the defect.The exact mechanism of formation of calcific bridge is not understood but it is hypothesized that the calcium hydroxide has the capability of inducing biotransformation of undifferentiated mesenchymal cells into odontoblasts to form a calcific barrier[1],[2].
As a liner & Base:
Resin based liners are applied in thickness of 25 micrometers below the restorations in dentin. This combination provides a protective film, providing better chemical insulation.
The primary functions of base are thermal insulation and to render mechanical support for the restoration by distribution of local stresses from the restoration across the underlying dentin surface. In addition to this, it seals the dentinal tubules thereby preventing the irritation of the pulp by leached chemicals through restoration[9],[10].
Apexogenesis:
In a vital immature tooth, the coronal pulp is amputated and covered with a suitable medication. The vitality of the radicular pulp is maintained and this allows root formation to proceed to completion. After root completion, conventional endodontic therapy is performed[9],[10].
Apexification:
Calcium hydroxide can be used as an intracanal medicament to induce the formation of hard tissue barrier at the apical 1/3rd of a non vital tooth following trauma when the root formation is still incomplete.
Cleaning and shaping is performed. Calcium hydroxide with a viscous vehicle is placed inside the root canal. It is left as an inter appointment dressing for 2-3 months and its monitored periodically, radiographically and clinically. After the hard tissue barrier forms, conventional endodontic therapy may be performed[11],[12].
Root Canal Sealer:
Several sealers Eg : Sealapex, CRCS, and Apexit have been marketed which claim benefits of the biological effects of added Ca(OH)2. In order to be therapeutically effective, calcium hydroxide must be dissociated into Ca2+ and OH- ions. Therefore, to be therapeutic an endodontic sealer based on Ca(OH)2 must release these ions which may affect the structural integrity of the sealer and compromise the long term seal.
In vivo studies demonstrated that seal apex and CRCS easily disintegrate in the tissue and both may cause chronic inflammation.Ca(OH)2 sealers are generally characterized as having good cyto compatibility[13],[14].
Intracanal Medicament:
Calcium hydroxide has been advocated as a routine intracanal dressing or when exudate persists or there is a long time interval between appointments[15].
Hard tissue induction in root fractures, root perforations and root resorption:
Root fracture
Intra-alveolar root fractures occur relatively infrequently, accounting for less than 3 per cent of all dental trauma. The fracture location influences the prognosis and treatment, and can be described as coronal, mid-root or apical third.
Andreasen and Hjorting-Hansen (1967) categorized possible spontaneous repair into four types:
1. Healing by calcification across a narrow fracture line.
2. Healing with intervening fibrous connective tissue.
3. Healing with root end resorption and replacement by wide bony in-till.
4. Persistent granulation tissue.
When a fracture occurs in the apical third treatment is often unnecessary since healing with calcified tissue interposed between the fragments is the usual sequela. Should periapical pathosis develop, endodontic treatment to the coronal segment and surgical removal of the fractured apical portion is usually successful. Cvek and Sundstrom (1974) advocated the use of calcium hydroxide between the two segments to induce a calcific barrier before obturating the coronal segment. However, this may take several months to occur[12],[13],[14],[15].
Root Perforations
Iatrogenic perforations can occur at the ‘elbow’ of a curved root canal during biomechanical preparation or during post hole preparation. A conservative technique to close the perforation with hard tissue induced by calcium hydroxide is possible if the defect is below the alveolar crest and is not in communication with the oral cavity[6],[14].
Root Resorption
Calcium hydroxide has been advocated for the management of both external and internal root resorption .
Internal root resorption that has not resulted in perforation is amenable to root filling techniques.
External resorption of either the inflammatory or replacement type may be arrested with the aid of a calcium hydroxide intracanal dressing prior to root filling (Cvek, 1981).
Weine (1989) described the use of calcium hydroxide paste placed into canals 3 weeks after tooth replantation in order to reduce external root resorption. However, the point is also made that if this is placed too soon, prior to periodontal ligament regeneration, resorption may be exacerbated. The basis for calcium hydroxide management of inflammatory root resorption relies on the pH change and neutralization of osteoclast produced acids, thus preventing mineral dissolution[6],[13].
Apical Plug
The intentional extrusion of calcium hydroxide powder to act as an apical stop itself, enabling condensation of guttapercha, has also been advocated with good clinical success rates[9],[10].
Conclusion
Ever since its introduction, calcium hydroxide has been extensively used in endodontics for vital pulp therapy, as intracanal medicament, apexification, apexogenesis, resorption, following replantation etc. Presently there is debate about the advisability of using calcium hydroxide for long term therapy. Further research is needed to resolve this issue.
References:
1. H.R.Stanley ,C.L.White & L.McCray. The rate of tertiary (reparative) dentin formation in human tooth. Oral Surg 1966;21:180.
2. M.Massler. Preventive endodontics: Vitalpulp therapy. Dental Clinics of North America 1967 ;11: 663-673.
3. G.S.Heithersay. Stimulation of root formation in incompletely developed pulpless teeth. Oral Surg. Oral Med. Oral Pathol. 1970;29: 620-630.
4. S.N.Frankl. Pulp therapy in Pedodontics. Oral Surg 1972; 34:293.
5. H.R.Stanley & Lundy. Dycal therapy for pulp exposure. Oral Surg 1972; 34:818.
6. L. R. Martin, B. Gilbert & A. W. Dickerson. Management of endodontic perforations. Oral Surg. Oral Med. Oral Patho. 1982;54: 668-677.
7. K. F Leinfelder., S. J. O’Neal and L. A Mueninghoff. Use of calcium hydroxide for measuring microleakage..Dent Mater.1986; 2: 121-124.
8. D.Orstavik .Antibacterial properties of endodontic materials. Int. Endod. J 1988; 21: 161-169.
9. P. C. Foreman & I. E. Barnes. A Review of Calcium Hydroxide. Int Endod J 1990;23:283-297.
10. A.Milosevic. Calcium hydroxide in restorative dentistry. J Dent 1991;19: 3-13.
11. L. R. G. Fava & W. P. Saunders. Calcium hydroxide pastes: classification and clinical indications. Int. Endod. J 1999;32: 257-282.
12. Walton Torabinejad.Principles of Endodontics.3rd Edition 2002:395-400.
13. S.Cohen, K.M. Hargreaves. Pathways of Pulp.9th Edition 2006: 839-840.
14. Ingle.Endodontic.6th Edition 2008: 1311-1313.
15. Weine. Endodontic Therapy.6th Edition 2009:227-228.
|