Introduction
Periodontal disease is a sequence of process from health to the formation of characteristic lesions, which includes periodontal pocket formation, loss of the gingival and periodontal connective tissue attachment and loss of tooth supporting alveolar bone[1]. Periodontitis is a progressive multifactorial disease whose course may be transient, undergoing severe periodontal destruction and remission of disease. Its activity is influenced by bacterial attack and which is responded by host response[2].
In response to bacterial attack there are triggering factors which are implicated to bring about the acute phase response; it is the chemical mediators of inflammation that plays an important role in the loss of connective tissue and supporting alveolar bone. The “acute phase reaction” represents an early and highly complex reaction of the individuals to a variety of injuries resulting in an elevation of the acute phase reactants such as α-2 macroglobulins, α-1antitrypsin, serum amyloid –A, haptogloblin and the most important C-reactive proteins (CRP) in Gingival crevicular fluid (GCF)[3].
C-reactive protein is a strong type 1 acute phase protein which is synthesized by hepatocytes and other cell types which include monocytes, endothelial cells, fibroblast and adipocytes. The discovery of C-reactive protein focused attention on the other acute phase response and the role of these proteins during an injury to the body[4].
Increased levels of CRP have been found in virtually disease associated with active inflammation or destruction[5],[6],[7]. CRP responds rapidly to inflammatory stimuli and serum level increases to several hundred folds. CRP is normally present in ng/ml quantities but may increase dramatically to hundreds of µg/ml within 72 hours following periodontal destruction. This represents an increase within hours of tissue damage, similar to antibody[4].
The released gingival inflammatory reaction results in elevated levels of CRP in gingival crevicular fluid[8] and in serum of the periodontitis subject[9]. The released CRP reacts with cell surface receptor, resulting in opsonisation; enhance phagocytosis and passive protection; activation of classical compliment pathway; scavenger for chromatin fragments; and modulation of pholymorphonuclear leukocytes function. The macrophage possess C-reactive protein receptors and thus CRP can potently up regulate pro inflammatory cytokine production (TNF, IL-1, IFN-γ, IL-8) aand anti inflammatory cytokine down regulates the acute phase proteins (IL-10, IL-4, IL-13, TGF-β). Thus elevated CRP levels serves as a non specific indicators for the presence of the disease process.
In the light of above mentioned facts, the present investigations are designed to estimate the levels of CRP in gingival crevicular fluid of subjects with healthy periodontium, gingivitis and periodontitis and to find out the correlation of CRP levels with incremental increase in inflammation.
Materials and Method
The study group consisted of 45 patients (25 males, 20 females aged 22-65 years) attending the outpatient section , Dept of periodontics, Govt dental college and research institute, Bangalore, Karnataka, India. Informed written consent was obtained from the patients who met the inclusion criteria and who agreed to participate in the study voluntarily. The inclusion criteria are, subjects who had not received any periodontal treatment in previous six months and presence of at least 20 functioning teeth. Ethical clearance was obtained from institutional ethical committee review board. Exclusion criteria for the study includes a) any history of systemic disease, b)subjects who have taken any medication( steroids, antibiotics & ant allergic drugs for at least 6 months prior to sampling procedure), c) subjects who have undergone oral prophylaxis or extraction for the last six months, d) individuals with habits of smoking and alcoholism.
Based on the gingival index (GI), probing pocket depth (PPD), clinical attachment level (CAL), and radiographic evidence of bone loss, subjects were categorised into three groups, Group I (HEALTHY) consisted of 15 subjects with clinically healthy periodontium with no evidence of disease with GI of 0, PPD≤ 3 mm, and CAL= 0 mm; there was no evidence of bone loss in the patients of this group. Group II (GINGIVITIS) consisted of 15 subjects whose gingival showed clinical signs of inflammation but there was no evidence of attachment loss and radiographic evidence of bone loss with GI › 1, PPD≤ 3mm & CAL= 0 mm; Group III (CHRONIC PERIODONTITIS) consisted of 15 subjects , who showed clinical signs of gingival inflammation and attachment loss with radiographic evidence of bone loss with GI ›1, PPD≥ 5mm and CAL ≥ 3 mm for this group.
GCF Collection
After the clinical and radiographic evaluation of the patients, they are segregated into different groups and sampling with selection was performed by one examiner. The site selected for sampling was dried isolated with cotton rolls to avoid contamination. Samples of GCF were obtained before probing in site by placing white colour coded 1-5 micro capillary pipettes which were obtained from Sigma- Aldrich chemical company (catalogue no P 0549). The test site was selected based on the periodontal status and GCF was collected. GCF was collected by gently placing the micro capillary pipette at the entrance of the gingival sulcus, care must be taken not to traumatize the gingival. Micro pipettes suspected of being contaminated with blood and/or saliva was excluded from the study. Each sample collection was allotted a maximum of 10 min, and the site did not express any GCF within the allotted time were excluded. This was carried out to ensure atraumatism. The GCF collected was immediately transferred to a plastic vial and stored at -700c till the time of assay.
ELISA Procedure
The collected samples were than assayed for C-reactive protein levels by using CRP-ELISA kit obtained from UBI- magiwellTM, USA ( Catalogue no AD-401). Samples were analyzed at department of Microbiology, Victoria hospital, Bangalore using Enzyme linked immunosorbent assay (ELISA method). The human CRP-ELISA can be used for quantitative determination of CRP making use of a solid phase sandwich ELISA. The wells (wells of conwing) are coated with specific antibodies directed towards human CRP. An aliquot of patient sample containing the endogenous patient CRP is incubated in the well with an enzyme conjugate of horse radish peroxidise. Enzyme conjugate forms sandwich complex with CRP bound to the well. The unbound conjugate is washed off with water. The amount of bound peroxidase is proportional to the concentration of the CRP present in the sample upon the edition of substrate and chromogen. The intensity of colour developed in proportional to the concentration of CRP. The intensity of colour developed was red on a spectrometer, with 450 nm as the primary wavelength. The level of CRP in the tested samples was estimated using the standard curve.
Statistical Analysis
All the data were analyzed using SPSS software (Version 14, 0; SPSS, Chicago, IL, USA). A non parametric procedure parallel to a one way analysis of variance is called Kruskul Wallis test was used. This approach is most useful when the samples data fail to fulfil the requirement of an analysis of variance. The mean of the ranks for each sample in each group was obtained by a Dunn’s multiple comparison test. Here parametric test were carried out for comparing the means of concentration in different groups.
Result
The present study was aimed at determining the association between c-reactive protein levels in GCF in periodontal health and disease. The three groups that constituted this study are health, gingivitis and chronic periodontitis. Each group consisted of 15 subjects from whom GCF was collected to estimate the concentration of CRP using ELISA. The highest mean concentration of GCF (1233.33ng/ml) were obtained from Group III and the least mean concentration for GCF (60 ng/ml) were obtained for Group I. For group II , the mean CRP level concentration for GCF (453.33 ng/ml) is intermediate as shown in (Table 1)
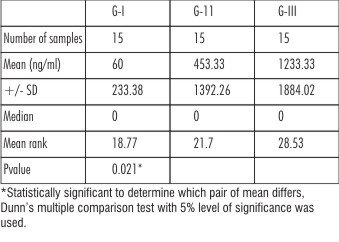 | Table 1 : I: Mean , Median ,SD And Kruskul- Wallis Test At 5% Level Of Significance
 |
As the means are not equal, a pair wise comparison of means was carried out using a Dunn’s multiple comparison tests. Here the absolute difference between mean ranks of any two group is compared with the critical value 9.39 obtained from the data. When group I- II and II-III were compared , the difference between mean ranks were 2.93 and 6.83 respectively which lesser than 9.39 being stastically insignificant. When group I and III were compared , the difference between mean ranks was 9.76 which is greater than 9.39being statistically significant. (Table II)
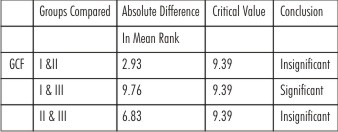 | Table 2 : Pair Wise Comparison Using Dunn’s Test For GCF
 |
Discussion
Pro inflammatory cytokines and mediators are significantly elevated with gingival inflammation during the destructive phase of periodontitis[10],[11][12][13][14][15][16]. The clinical findings in periodontitis have emphasized the local nature of inflammation and tissue destruction within the oral cavity. One consequences of these localized gingival inflammatory reaction has been the identification of elevated levels of various acute phase proteins in the GCF [10], [17], [18]. The level of CRP a type of acute phase protein which is altered in crevicular environment, presumably present as a result of numerous host bacterial interactions in the sulcus and may contribute to the defence of the host in this milieu.
Most studies of periodontitis have emphasized the local nature of this host bacterial interaction in the periodontium and gingival sulcus[11],[12],[16],[19], it also appears that systemic manifestation of this disease are also detected to many oral bacteria and appears to be increased with periodontitis[20],[21] potentially resulting from transient access of oral bacteria to the circulation[22]. Therefore the ability to use acute phase reactants level as a measure of infectious process or inflammatory disease has substantial support.
The quality the microbial ecology confirms the higher probability of the localized infection[23], or that there exists a subset of high risk patient with increased susceptibility attributed to attend local inflammatory response characteristics. The alternatives could have important implication for host bacterial variations which contributes to increased succesaptibility to resistant to periodontitis progression. Thus measurement of CRP in GCF may help to identify a subset of patients who are at high risk for destructive disease, or disclose those patients who are undergoing a process of periodontal breakdown.
The importance of quantifying CRP in GCF may have potential diagnostic tests and to evaluate potential protein as indicators of disease. Although CRP is produced by hepatocytes there is an evidence for being present on cell associated with acute connective tissue inflammation[24]. The rapid rise of CRP in GCF following exposure to IL-1 which is a potent bone resorber also found in GCF, made the search for elevated CRP levels in inflamed periodontal tissue seen reasonable.
The result of the present study are in agreement with that of the Ebersole et al and Noack, B et al. The mean GCF concentration of CRP in periodontitis group was very much higher than the gingivitis group and healthy group. Thus the mean concentration increased progressively from health to gingivitis to periodontitis in GCF.
However, the results are in contrast to P.D.Sibra et al, who found that the CRP levels in GCF could not vary significantly between health and disease sites. Periodontal disease sites exhibited lower level of CRP; which could be attributed to the different experimental deigns and difference in sensitivity of direct and indirect immunodot technique were evaluated for quantifying CRP in GCF.
The wide range observed in the CRP levels in gingivitis, periodontitis subjects could result in part from differences in disease activity and crevicular fluid flow at the gingival sites, as well as from variation in the number of polymorphonuclear neutrophils migrating into the crevice. Although there was a small intra group variations observed which might reflect individual variation in plaque accumulation or gingival inflammation in response to plaque. Alternatively it could reflect an inherent variations in the local periodontium . There may be differences between individuals in the course and resolution of the inflammatory process and the resulting vascular permeability. These facts are in agreement with the study of E.Adanogianaki at al.
The CRP level found in healthy subjects and absence in diseased sites may be because of many of our diseased sites were probably stable, and some healthy sites may have been undergoing active attachment loss. Thus the value of CRP as an indicator of active periodontitis must therefore await longitudinal and prospective study.
In summary the greater amount of periodontal tissue destruction, the higher is the mean CRP concentration. But there is a high degree of variability of CRP concentration from one subject to the other in the different groups. Although CRP levels can be considered as a “prognostic biochemical marker” of periodontal tissue destruction, there inter subject variation should be nullified by further studies carried out on the same subjects with health sites progressing on to gingivitis and then to periodontitis.
The mean CRP concentration level increases significantly as the progression of disease occurs with greater periodontal destruction. However, further research is required t use CRP levelas a “Biomarker” of periodontal disease and its influence on systemic disorders
References
1. Robert J. Genco, Henry M. Godman, D. Walter cohen. Contemporary periodontics, 6th ed; 1990:27.
2. Cripps. Periodontal disease: recognition, interception and prevention, 4th ed. Mosby ; 1984:36.
3. Sibraa P.D., Reinhardt R A., Dyer J. K., et al. Acute phase protein detection and quantification in gingival crevicular fluid by direct and indirect immunoblot. J clin periodontol 1991;18:101-106.
4. Jeffrey L., Ebersole., David capell. Periodontol 2000(23):19-49.
5. Hedlund P et al. Clinical and experimental studies on C-reactive protein (acute phase protein). Acta med scand 1961; 361:1-71.
6. Anderson H.C., Mc Carty. Determination of C-reactive protein in the blood as a measure of the activity of the disease process in acute rheumatic fever. Am j Med 1950; 8: 445-455.
7. Kroop I.G., Shackman N.H. et al. Levels of C-reactive protein as a measure of acute myocardial infection. Proc soci exp boil med 1954;86:95-97.
8. Adonogianaki E., Moughal N.A., Stirrups D., Kinane D.F., Acute phase protein in gingival crevicular fluid during experimentally induced gingivitis.J periodont Res 1994;29:196-202.
9. Ebersole J.L., Machen R.L., Steffen M.J., Willmann D.E. Systemic acute phase reactants, C-reactive protein and haptoglobein in adult periodontitis. J exp immunol 19997;107:347-352.
10. Tuck C. H., Holleran S., Berglund L. Hormonal regulation of lipoprotein (a) level; effects of estrogen replacement therapy on lipoprotein (a) and acute phase reactants in post menopausal women. Arterioscler thromb vasc boil 1997; 17:1822-1829.
11. Page R.C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J periodont Res 1991;26: 230-242.
12. Lamster I.B., Novakk m.J. Host mediatiors in gingival crevicular fluid: implications for the pathogenesis of periodontal disease.Crit Rev Oral Biol Med 1992;3:31-60.
13. Ebersole J.L., Singer R.E., Steffensen B., Fillon T., Kornman K. S. Inflammatory mediators and immunoglobulins in GCF from healthy, gingivitis, and periodontitis sites. J Periodont Res 1993;28:543-556.
14. Tonetti M.S., Freiburghaus K., Lang N.P., Bickel M. Detection of interleukin-8 and matrix metalloproteinases transcripts in healthy and diseaded gingival biopsies by RNA/PCR. J Periodont Res 1993; 28:511-513.
15. Bickel M. The role of interleukin-8 in inflammation and mechanism of regulation. J Periodontol 1993;64:456-60.
16. Ranney R. R. Immunologic mechanisms of pathogenesis in periodontal diseases: an assessment. J Periodont Res 1991; 26:243-54.
17. Yother J., Volanakis J., Briles D.E. Human C-reactive protein is protective against fatal streptococcus pneumonia infection in mice. J Immunol 1982;128:2374-2376.
18. Ebersole J.L., machen R.L., Steffen M.J., Willmann D.E. Systemic acute phase reactants, C-reactve protein and haptoglobin, in adult periodontitis.Clin Exp Immunol 1997; 107:347-352.
19. Seymour G.J., Germell E., Reinhardt R.A., Eastcott J., Taubman M.A., Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodont Res 1993;28:478-486.
20. Ebersole J.L. Systemic hormonal immune responses in periodontal disease. Crit Rev oral Biol med 1990 ;1:283-331.
21. Trautwein C., Boker K., Manns M.P. Heptocyte and immune system: acute phase reaction as a contribution to early defense mechanisms. Gut 1994;35:1163-1166.
22. Holister M.C., Weintraub J.A. The association of oral status with systemic health, quality of life, and economic productivity. J Dent Educ 1993;57:901-912.
23. Haffajee A.D., Socransky S.S., Microbial etiological agents of destructive periodontal disease. Periodontology 2000; 1994:5:78-111.
24. Gewurz, H., Mold C., Seigal J and Feidel B. C-reactive protein and the acute phase response.Adv intern med 1982;27: 345-372.
|