Introduction
There has been a great increase in knowledge of the pathology of odontogenic cysts in recent years, so it is an opportune moment to review the advances that have been made. It is not possible to define the reason for this upsurge in interest, but if there is one observation that has spurred it on, it must be the evaluation of the significance of the structure of the epithelial lining of odontogenic cysts, particularly the presence or absence of a keratin layer.[1]
The significance of keratin formation in the epithelial lining of odontogenic cysts is two fold: firstly, the great majority of these cysts fall into a category i.e. the odontogenic keratocyst, which exhibits a more troublesome pattern of clinical behaviour than the other cysts and secondly keratinizing cyst is more common amongst the cysts which have undergone carcinomatous change.[1]
Odontogenic Keratocyst (OKC) was first described in 1876, and named by Phillipsen in 1956. It is one of the most aggressive odontogenic cysts of the oral cavity. OKC is known for its rapid growth and its tendency to invade the adjacent tissues including bone. It has a high recurrence rate and can be associated with the basal cell nevus syndrome.[2] OKCs are generally thought to be derived from either the epithelial remnants of the tooth germ or the basal cell layer of the surface epithelium.[3] The majority of the patients fall in the age group between 20 to 60 though it can occur in any age varying from the very young to the very elderly. The male to female ratio is 1.44:1 (Brannon);[4] though variations have been reported .OKC may occur in any part of the upper jaw and lower jaw, the majority occurring in the mandible, most commonly in the angle of the mandible and ramus.[5]
Orthokeratinized odontogenic cyst (OOC) was first described by Schultz[6] in 1927 as a dermoid cyst, and in 1945, it was considered by Philipsen[7] as an eventual type of odontogenic keratocyst (OKC). In 1981, Wright[8] specified its clinicopathologic aspects, assuring that OOC was an individual entity, distinct from other odontogenic cysts, including the OKC. After that, a considerable number of studies have discussed the real nature and histopathologic classification of OOC. OOC has been described as a solitary lesion, usually small and nonexpanding, radiolucent and asymptomatic, usually occurring in the posterior mandible between the fourth and fifth decades without gender predilection. OKC occurs mainly in the second and third decades with a mild predilection for men. Usually, OKCs are single lesions, unless they are associated with basal nevoid syndrome. In contrast to OKCs, the OOC has no tendency to recur and is not associated with the nevoid basal cell carcinoma syndrome.[9]
OKC, now also known by a new term “keratocystic odontogenic tumor” (KCOT) which was first given by Reichart in 2005.[10] The discovery of increased mitotic activity in cyst epithelium, potential for epithelial budding from basal layer or daughter cysts in cyst wall, presence of chromosomal abnormalities, role of mutation in PTCH gene in the etiology of KCOT resulted in reclassification of OKC as a neoplasm in World Health Organizationclassification of head and neck tumors in 2005 and its renaming as KCOT.[11]
Even though immunohistochemistry and molecular techniques have been widely used for investigative procedures, they have had a very little impact in demonstrating the cystic fibres and traditional histopathology continues to be the mainstay for the diagnosis together with the histochemical stains. In order to demonstrate as well as compare the cystic lining and capsule of OOC and OKC the gold standard i.e. hematoxylin and eosin staining , picrosirus staining and IHC with Ki67 was done in the present study.
Materials And Methods
All biopsy specimens diagnosed as odontogenic keratocyst were again reviewed from the archives of Department of Oral Pathology. Approximately 20 cases previously diagnosed as Odontogenic keratocyst showing epithelium lined cavity in which a significant proportion of the keratinization was seen were included in the present study. Inflammatory cysts showing focal keratinization within the epithelial lining were excluded because it has been speculated that focal keratinization may represent metaplasia.
Ten cases of OOC and ten cases of OKC were retrieved from the Department of Oral Pathology at Govt Dental College, Rohtak. Criteria for selection of the OOCs were based on those established by Wright[8] i.e. thin uniform epithelial lining with a luminal surface of orthokeratin, a well developed granular layer, and a basal cell layer containing flattened squamous or cuboidal cells. Philipsen’s criteria for identifying OKC was uniform epithelial lining ranging from 6 to 10 cells thick with a well developed palisaded , polarized basal cell layer and a luminal surface of corrugated parakeratin.[10]
Samples
This laboratory based study involved the use of archival tissue i.e. buffered formalin fixed, paraffin embedded tissues of previously histopathologically diagnosed cases of OKC from the archives of Department of Oral Pathology. For the purpose of this study, samples were divided into two groups. Group A was OKC with parakeratinized epithelial lining. Group B was of OOC having orthokeratinized epithelial surface
Group A - OKC -10
Group B - OOC - 10
All the samples in both the groups were stained using routine Hematoxylin and eosin stain.
Immunostaining was carried out with the peroxidase – labeled EnVision system on dewaxed tissue sections. Sources, clones, concentrations, incubation periods of the primary monoclonal antibody and antigen retrievals used for the antibody have been summarized in Table 1. The antibody used was antihuman Ki67 antigen clone i.e. MIB1 monoclonal primary mouse RTU antibody (Dako cytomation). Normal oral mucosa was used as positive control. Negative controls were obtained with incubation of the sections in the tris-HCl buffer (pH, 7.4) instead of the primary antibody.
 | Table 1. Monoclonal Antibodies Used
 |
Many histochemical stains have been used to demonstrate collagen fibres like Van Gieson, Masson’s trichrome, Weigert’s Resorcin Fushsin, modified Movat’s stain, Goldner’s Trichrome method, Wilder Modification of Bielschowsky’s method etc.[19],[20], but the picrosirius red stain under the polarizing microscope is the most widely used because of the inherent nature of birefringence of collagen.
For the standardization purpose, five mucosal tissues were included to assess the color taken up by the normal fibres. Sections of 5 µm thickness were made from each block of study and control groups. They were stained with both Hematoxylin and Eosin and by modified Picrosirius red procedure. H& E stained section was assessed for the diagnosis of the cyst. The picrosirius red stained section was assessed using the ocular micrometer under oil immersion; the thickness of the fibres along with the color exhibited by them were noted. Around 50 fibres were measured in each group and was categorized as thin (< 0.8 µm) and thick fibres (1.2 µm - 2.4 µm) and the color of fibres were categorized as greenish yellow or orange red. Overlapping of the colors or the thickness of fibres was not taken into account.
Results
Microscopic examination of the cysts stained with hematoxylin and eosin showed that OOCs were formed by a fibrous capsule lined by a uniform epithelium 4 to 8 cell layers thick. This epithelium was covered by a thick layer of orthokeratin and the granular layer was also prominent. The basal layer was composed of cuboidal or flattened cells, and no palisaded or polarized nuclei were seen (Fig 1). OKC capsules were lined mostly with parakeratinized epithelium with a demarcated basal layer formed by columnar or cuboidal palisaded cells whose nuclei were usually polarized (Fig. 2). The luminal epithelium was covered with orthokeratin in every case of Group B i.e. OOC. The predominant histopathologic picture was one of orthokeratinization with a subjacent granular cell layer. No attempt was made to quantitate the granular cell layer since it was variable. Only in one case orthokeratin was found without an underlying granular layer. In every case of Group A the epithelium was thin uniform in the areas of keratinisation. The epithelial thickness averaged from 6-8 cells. Out of ten cases in group A three cases showed areas of ulceration, rete ridge formation was infrequent.
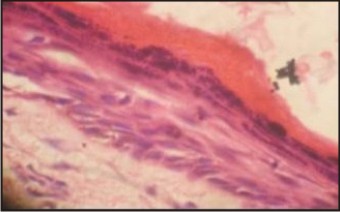 | Figure 1
 |
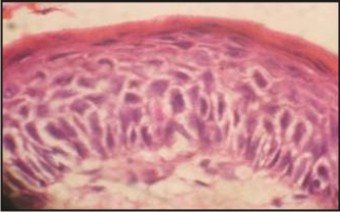 | Figure 2
 |
In group A the cyst showed a tendency to involve the posterior jaw i.e the region distal to and including first molar. Out of ten cases , 2 cases involved only the mid body i.e premolar area. Three cases showed the involvement of mandibular third molar area. All the cysts in Group B i.e. OOC showed a tendency to involve mandibular posterior region with involvement of mandibular third molars also.
Picrosirius red stain with polarizing microscopy had been used to study the individual collagen fibres and the color exhibited by these fibres depends on fibre size, alignment and packing of fibres, molecular organization, ground substances and water content. Normally, green to greenish yellow corresponds poorly packed fibres, whereas orange red represents well packed fibres[21],[22].
Extracellular matrix components showed different patterns of expression between the 2 cysts studied. In OOC collagen fibres were diffusely arranged with few immature fibres at the periphery of connective tissue capsule. (stained fibrils and fibroblasts) aspect (Fig. 3). In OKC Picrosirius red staining showed collagen fibres compactly with more immature green fibres at the periphery of connective tissue capsule (Fig 4).
 | Figure 3
 |
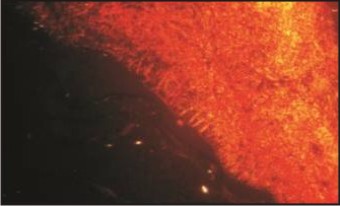 | Figure 4
 |
Statistical analysis was done to determine the significance of these findings observed between the thickness of the fibres among the cyst groups and to see the effect of inflammation on the color of the fibres. Mean and standard deviation were calculated for the individual groups. They were compared using the following test of significance: T test.
Assessment of Ki67 IHC staining was done using Image Pro image analysis software version 5. Ki67 immunoreactivity was assessed in areas of highest staining intensity. Atleast 1000 nuclei were collected in 5 high power fields (x400). The Ki67 labelling index (number of positive cells/ total cells x 100) were calculated for both groups. The mean of Ki67 labelling index were submitted to statistical analysis using the Kruskal- Wallis H test. A p value of less than 0.05 was considered statistically significant. Ki67 positive cells in OKCs were observed in basal and predominantly suprabasal cells. However, OOC did not show much of Ki67 expression. Number of positive Ki67 nuclei in OKC (n=10) was 10.7+_ 3.3%) ( mean +_ SD). OOC showed 4.5+_ 2.1% Ki67 nuclei.
Discussion
The precise incidence of OOC is unknown because criteria adopted for characterizing the cyst are not uniform in the pertinent literature.[12] According to Siar and Ng[13] percentage indices vary between 3% to 11%, and Li et al[14] showed a variation between 5.2% to 16.8%. However, despite nonstandardized studies, clinicopathologic aspects such as age of incidence, biologic behavior, and immunohistochemical aspects, described for OOC in the various studies, are quite similar, and different from those described for OKCs.[15],[16] Histologic criteria adopted in this study, on the basis of those proposed by Wright[8] in 1981, provided a rapid and precise diagnosis of OOC, allowing its distinction from areas of orthokeratinization that, although rare, may appear in OKC. The distinction between OOC and OKC is important because OKCs have a marked tendency to recur and may be part of the nevoid basal cell carcinoma syndrome, and although it is unlikely, neoplastic transformation may occur.[13]
Recent immunohistochemical studies that compared OOCs with parakeratinized OKC have shown distinct differences in the expression of Ki67 proliferation index. Variable expression of IHC markers of OKC & OOC[17] reflect the variation in epithelial cell maturation, proliferation between two types of epithelial lining, those of OOC seem to assume a different cell differentiation and exhibit lower cellular activity than those of OKC. Wysocki and Sapp showed that there are distinct ultrastructural differences between OKCs and OOCs.[18]
Conclusion
From the results of this study, it would appear that the orthokeratinized variant of OKC is histopathologically and clinically distinct from the more common parakeratinized variant. Therefore , the orthokeratinized variant of OKC warrants its distinct recognition. Although the histogenesis of OKCs has been debated, most authors believe that they originate from dental lamina. This would explain their common occurrence in the posterior mandible, because the dental lamina is more active in this area at the age when many patients develop their cysts. This theory would also support an extrafollicular development for dentigerous cysts.OKCs have been studied extensively with respect to their aggressive clinical behaviour. This has been ascribed to high mitotic index and high turnover rate for OKC epithelium, osmolarity of cyst contents, active collagenase and prostaglandin concentration. Although none of the parameters was specifically studied in this study, the mitotic rate of orthokeratinized epithelium was subjectively interpreted as low. The smaller size of orthokeratinized OKCs as compared to parakeratinized cysts could be explained by its decreased aggressive behaviour.
References
1. R.M. Browne. The pathogenesis of odontogenic cysts: a review. Journal of Oral Pathology 1975,4 : 31-36.
2. Oda Dolphine et al. Odontogenic keratocyst: The Northwestern USA experience. Journal of Contemporary Dental Practice 2000, 1 : 1-8.
3. Hjorting-Hansen E, Andreasen JO, Robinson LH. A study of odontogenic cysts with special reference to location of keratocysts. Br J Oral Surg 1969;7: 15-23.
4. Brannon RB. The odontogenic keratocyst. A clinicopathologic study of 312 cases. Part II. Histologic features. Oral Surgery 1977;43: 233-55.
5. Haring JI, Van Dis ML. Odontogenic keratocysts; a clinical, radiographic and histopathologic study Oral Surg Oral Med Oral Pathol 1988;66: 145-53.
6. Schultz L. Cysts of the maxillae and mandible. J Am Dent Assoc 1927;14:1395-402.
7. Philipsen HP. Om keratocyster (kolesteatom) i kaeberne. Tandlaegeblated 1956;60:963-81.
8. Wright JM. The odontogenic keratocyst: orthokeratinized variant. Oral Surg Oral Med Oral Pathol 1981;51:609-18.
9. Crowley FE, Kaugars GE, Gunsolley JC. Odontogenic keratocysts: a clinical and histologic comparison of the parakeratin and orthokeratin variants. J Oral Maxillofac Surg 1992;50:22-6.
10. Philipsen HP. Keratocystic odontogenic tumour. In: L Barnes, J Eveson, P Reichart, D SidranskyWHO classification of tumours. Pathology and genetics of tumours of the head and neck. International Agency for Research on Cancer (IARC) Lyon. 2005; 306–307.
11. Habibi A, Saghravanian N, Habibi M, Mellati E, Habibi M. Keratocystic odontogenic tumors: a 10 year retrospective study of 83 cases in an Iranian population. Journal of Oral Science 2007 ; 49 (30): 229-235.
12. Karantza-Angelopoulou E, Nicolatou O. Opinions regarding: odontogenic keratocysts. J Oral Maxillofac Surg 1990;48:1353-4.
13. Siar CH, Ng KH. Orthokeratinized odontogenic keratocysts in Malaysians. Br J Oral Maxillofac Surg 1988;26:215-20.
14. Li TJ, Kitano M, Chen XM, Itoh T, Kawashima K, Sugihara K, et al. Orthokeratinized odontogenic cyst: a clinicopathological and immunocytochemical study of 15 cases. Histopathology 1998;23:242-51.
15. Vuhahula E, Nikai H, Ijuhin N, Ogawa I, Takata T, Tanimoto K.Jaw cysts with orthokeratinization analysis of 12 cases. J Oral Pathol Med 1993;22:35-40.
16. Karantza-Angelopoulou E, Nicolatou O. Opinions regarding: odontogenic keratocysts. J Oral Maxillofac Surg 1990;48:1353-4.
17. Thosaporn W, Lamaroon A, Ponqsiriwet S, Nq KH. A comparative study of epithelial cell proliferation between the odontogenic keratocyst, orthokeratinized odontogenic cyst, dentigerous cyst, and ameloblastoma. Oral Diseases 2004; 10(1) : 22-6.
18. Da Silva MJ, de Sousa SO, Correa C, Carvalhosa AA, De Araujo VC. Immunosistochemical study of the orthokeratinized odontogenic cyst: a comparision with odontogenic keratocyst. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002 ; 94 (6) 732-37.
19. Bancroft J D, Gamble M. Theory and practice. 6 ed. Churchill Livingstone: Elsevier; 2008: 135-159.
20. http://www.emsdiasum.com / microscopy / products / histology / connective_tissue_stains.aspx
21. Dayan D, Hiss Y, Hirshberg A, Bubis J J, Wolman M. Are the polarization colors of Picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry and Cell Biology 1989; 93(1):27-29.
22. Junqueira L.C. Montes G S, Sanchez. The influence of tissue thickness of collagen by the Picrosirius – Polarization method. Histochemistry 1982; 74 (1): 153- 6.
|