Introduction
Though routinely reported in literature since 180 years[1], there is a continuing paucity regarding the incidence and prevalence rates of exostoses, typically torus mandibularis in the published texts. These bony hamartomas are asymptomatic, benign, exophytic nodular outgrowths of dense cortical bone[2] that are relatively avascular. Maxilla is shown to exhibit the highest prevalence rate of 5.1:1 in comparison to mandible with male population afflicted more than females 1.66:1, in all intraoral locations[3]. Usually the bicuspid and molar areas are the affected sites yet occasionally they may occur in other parts of the jaw, either as a smooth bulging of the bone surface continuous with the adjacent area or as discrete, multilocular spherical projections with a broad base that forms a nodular cluster[4],[5]. Buccal surface involvement however, typically in the mandible has been an unusual occurrence.
Commonly presenting as solitary discreet masses of varying proportions, in the rare instances of untoward sporadic exacerbations, they may present as colossal unaesthetic masses, creating speech issues due to limited tongue space. Additionally, they may contribute significantly towards the inception of periodontal disease by promoting food lodgement[6]. Histologically, the soft tissue overlying the exostosis has been found to be thinner than the gingiva thus predisposing it to trauma or ulceration when masticating hard or sharp food[7]. Successful recording of dental impressions and seating of complete as well as partial dentures have posed a constant therapeutic challenge in such patients. No malignant potential has been reported till date.
With the growing emphasis on cosmetic dentistry and esthetics especially among the youngsters, surgical removal is routinely warranted for such lesions. This article will report a rare case of multiple mandibular exostosis along the anterior labial region along with its surgical management.
Case Description
A 33 year old female patient reported to our dental office with the chief complaint of unaesthetic facial appearance owing to the increased lower jaw size. On clinical examination, multiple bony projections along the buccal surface of the jaw in the anterior region, at a level below the mucobuccal fold could be visualized. Overlying mucosa appeared to be stretched thus blanched with focal areas of inflammation and traumatic ulcerations. Keeping in mind the predicament of the patient and a desire for an esthetic form, surgical intervention for complete excision was planned. Routine blood and urine investigations were found to be non- contributory. After explaining the patient the potential risks and benefits, an informed consent was obtained. Once adequate anesthesia was achieved, a full thickness mucoperiosteal flap was elevated to gain complete access to the exostosis. Following exposure of the lesion, a molt periosteal elevator was placed at the apical end to prevent inadvertent injury during excision. The bony growth was sequentially reduced from the superior border employing a micromotor diamond bur under copious saline irrigation. Final step involved the complete alveolar gradualization to mimic the physiological bone contour. Mucoperiosteal flap was trimmed to fit the re-established bony outline and secured with black silk sutures. Postoperatively patient was appropriately prescribed medication and chlorhexidine mouth wash. No obvious post-operative complications were observed. The patient was scheduled to report back in a week’s time for post- operative follow-up and suture removal. Uneventful healing at the surgical site with no untoward discomfort was reported by the patient.
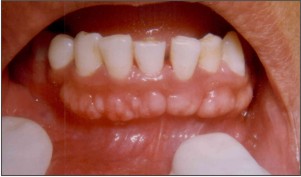 | Fig.1. Multiple buccal exostoses in mandibular anterior region: Pre-operative View
 |
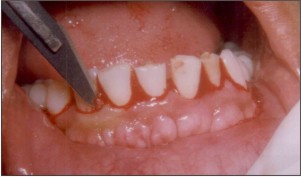 | Fig.2. Intra sulcular incision to gain access to the surgical site
 |
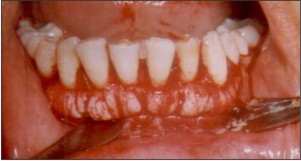 | Fig.3. Full thickness mucoperiosteal flap raised to expose the exostoses
 |
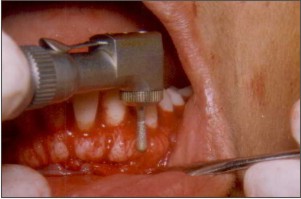 | Fig.4. Scoring of the bony exostosis being done with a micromotor diamond bur
 |
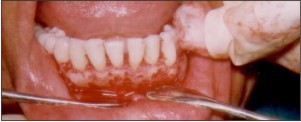 | Fig.5. Surgical site following restitution of anatomical contours
 |
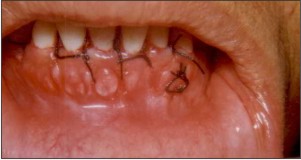 | Fig.6. Surgical site after placement of sutures
 |
Discussion
Meager representation of 0.09% among the contemporary constitutional bony disorders merits exostosis as an uncommon benign tumor of the oral cavity. The novelty of this article lies with the fact that the bony outgrowth in question was found along the buccal aspect of the mandible in a female patient; all factors emphatically documented in literature of least predilection.
Several authors have attempted to investigate the etiology of this bony hamartoma though no consensus has been reached. A multifactorial etiology encompassing a complex interplay of genetic and environmental factors has been strongly implicated in the advent of the lesion[8],[9]. Giving credence to the quasi-continuous genetic or threshold theory, Gould AW(1964)[10] identified exostosis as an autosomal dominant lesion expressed in the event when the environmental factors reach their threshold levels. Variation in frequency and morphology among various racial and ethnic groups also strongly suggest a genetic basis of the lesion attributable presumably to the variation among groups in diet and food preparation habits[11],[12]. Curiously, literature documents cases of spontaneous sequestration of bone occurring with an almost certainty in patients genetically predisposed to develop exostosis.
A cumulative functional influence on the alveolar bone particularly in the elderly patients has been positively correlated to an increased prevalence of exostosis[13] in this strata of patients as identified by Nery et al[14]. The bony outgrowths are thought to represent a reaction to increased or abnormal occlusal stress to the teeth in the involved areas[15]. Clinical research has extensively supported this hypothesis based on significant co-occurrence of exostosis in patients with para-functional habits viz. bruxism, clenching and grinding[16],[17]. Jaws deformed due to bony buttress formation in an attempt to ward off excessive occlusal forces in these patients provide the clinical evidence for the same. Severe occlusal wear alters the masticatory plane and changes the distribution of strain and pressure forces along the alveolar bone and this redistribution exerted particularly by molars has been experimentally shown to induce bone remodeling in rat maxillas[18]. Sonnier et al[19] in contrast however reported a decreasing prevalence beyond 50 years of age correlating it with edentulism resulting in gradual remodeling and its subsequent obliteration[20]. A diminished masticatory demand after the third and fourth decades of life appears to be underlying mechanism.
A remarkably strong pattern of concurrence of exostosis, torus palatinus and torus mandibularis has been recognized[3] in literature which persuasively suggests general multiple exostosis syndrome.
Interestingly, van den Broek (1941)[21] incriminated chemical irritation of the alveolar bone as witnessed in periodontal disease with subsequent mineralisation of the inflamed fibrous tissue, as a possible etiological factor for exostosis. Buccal exostoses have also exhibited a significant correlation with caries and calculus occurrence, calculus severity and a particularly strong association with periodontal disease as demonstrated by Pechenkina and Benfer[22].
Ossenberg[23] proposed the etiologic role of root apex pressure on the periodontal ligament chiefly in the incidence of mandibular exostosis. Lingually tilted roots of upper molar and buccally tilted lower molar roots is known to exert pressure on the periodontal ligament in opposite directions resulting in micro ruptures in the ligament thereby initiating periodontal infection triggering exostosis formation.
Schriener[24] postulated vitamin deficiencies leading to damaging of connective tissue and frequent haemorrhages as yet another possible causative factor for exostosis in genetically susceptible individuals.
For successful therapeutic intervention, it is therefore imperative to correctly diagnose the contributing factors to allow complete resolution of the lesion.
Conclusion
Although a multifactorial etiology has been established, yet cosmetic and functional demands of the patients necessitates the surgical excision of such bony excrescences with a concomitant attempt to limit the underlying etiological factors as much as possible.
Conflict Of Interest
There was no financial support in the form of grants, equipments, drugs or any other significant sources of support for the present study. Also there was no financial relationship between the authors and any commercial firm.
References
1. Shah DS, Sanghavi SJ, Chawda JD et al. Prevalence of torus palatinus and torus mandibularis in 1000 patients. lndian J Dent Res. 1992:3:107-l10.
2. Pynn BR, Kurys-Kos NS, Walker DA, et al. Tori mandibularis: a case report and review of the literature. J Can Dent Assoc. I995;6I:1057,1058.1063-106
3. Jainkittivong A, Langlais R. Buccal and palatal exostoses: prevalence and concurrence with tori. Oral Surg Oral Med Oral Pathol O ral RadioI Endod. 2000;90 :48 -53.
4. Stephen TS, Robert CF, Leslie F. Philadelphia: WB Saunders Company; 1984. Principle and Practice of Oral Medicine; pp. 476–7.
5. Bruce I, Ndanu TA, Addo ME. Epidemiological aspects of oral tori in a Ghanaian community. Int Dent J. 2004;54:78–82.
6. Gorsky M, Raviv M, Kfir E, Moskona D. Prevalence of torus palatinus in a population of young and adult Israelis. Arch Oral Biol 1996;41:623-5.
7. Kurtzman GM, Silverstein LH, Shatz PC. A Technique for Surgical Mandibular Exostosis Removal.CompendiumOctober 2006;27(10):520-525.
8. Blakemore JR, Eller DJ, Tomaro AJ. Maxillary exostosis. Oral Surg Oral Med Oral Pathol 1975;40:200-4.
9. Seah YH. Torus palatinus and torus mandibularis: a review of the literature. Aust Dent J 1995;40:318-21.
10. Gould AW. An investigation of the inheritance of torus palatinus and torus mandibularis. J Dent Res 1964;43:159-67.
11. Reichart PA, Neuhaus F, Sookasem M. Prevalence of torus palatinus and torus mandibularis in .Germans and Thais. Community Dent Oral Epidemiol 1998;16: 61–64.
12. King DR, King AC. Incidence of tori in three population groups. J Oral Med 1981;36: 21–23.
13. Shear M, Speight P. Cysts of the Oral and Maxillofacial region. Blackwell Publications Ltd.;2007:6-58.
14. Nery EB, Corn H, Eisentein IL. Palatal exostosis in the molar region. J Periodontol 1997;48:663-6.
15. Regezi JA, Sciubba JJ. Oral pathology: clinico-pathologic correlations. Philadelphia: WB Saunders Co; 1989. p. 386-7.
16. Sirirungrojying S, Kerdpon D. Relationship between oral tori and temporomandibular disorders. Int Dent J 1999;49: 101–104.
17. Kerdpon D, Sirirungrojying S. A clinical study of oral tori in southern Thailand: prevalence and the relation to parafunctional activity. Eur J Oral Sci 1999;107: 9–13.
18. Waldo CM, Rothblatt JM. Histologic response to tooth movement in laboratory rat. J Dent Res 1954;33:481-86.
19. Sonnier KE, Homing GM, Cohen ME. Palatal tubercles, palatal tori and mandibular tori: prevalence and anatomical features in a US population. J Periodontol 1999;102:60-3.
20. Axelsson G, Hedegård B. Torus palatinus in Icelandic schoolchildren. Am J Phys Anthrop 1984;67:105–112.
21. Van den Broek AJP. On exostoses in the human skull. Acta Nederland Morph 1941; 5: 95–118.
22. Pechenkina EA, Benfer RA. The role of occlusal stress and gingival infection in the formation of exostoses on mandible and maxilla from Neolithic China. HOMO 2002;Vol. (53/2):112–130.
23. Ossenberg NS. Mandibular torus: a synthesis of new and previously reported data and discussion of its cause. In Cybulski JS (ed) Contribution to Physical Anthropology 1978–1980. National Museum of Canada, Ottawa, 1–52.
24. Schreiner KW. Zur Osteologie der Lappen. Instituttet for Sammenlignende Kultforskning, Series B 1935;18:161–177.
|