Introduction
Inflammatory jaw cysts comprise a group of lesions that arise as a result of epithelial proliferation within an inflammatory focus due to a number of causes. Radicular cysts are the most common inflammatory cysts and arise from the epithelial residues in the periodontal ligament as a result of periapical periodontitis following death and necrosis of the pulp. Cysts arising in this way are found most commonly at the apices of the involved teeth, but may also be found on the lateral aspects of the roots in relation to lateral accessory root canals. Quite often a radicular cyst remains behind in the jaws after removal of the offending tooth and this is referred to as a residual cyst.[1]
About 60% of all odontogenic cysts are comprised of radicular and residual cysts.[2] These cysts can occur in all tooth bearing areas of jaws, although more frequent in maxillary than mandibular teeth.[1] Maximum number of cases are seen in 4th and 5th decade and a slight male predilection is seen.[2] Most of the radicular cysts are symptomless unless infected.
Histologically, it is a true cyst and is lined wholly or in part by stratified squamous epithelium supported by fibrous connective tissue wall. About 10% of the radicular cyst shows presence of eosinophilic bodies known as hyaline or rushton bodies in the epithelial lining of the cyst. Very rarely they are present in the fibrous capsule. The bodies measure up to about 0.1mm and are linear, straight or curved or of hairpin shape and sometimes they are concentrically laminated. They take eosin stain in routine hematoxylin and eosin (H&E) stained sections. Circular or polycyclic bodies are also seen with a clear outer layer surrounding a central granular body.[1]
Here, we present a case describing presence of two radicular cysts in a patient that showed presence of rushton bodies within the epithelial linings of both the cysts.
Case Report
A 32 year old female patient reported to the outpatient department of our institute with a chief complaint of pain and pus discharge in upper left front tooth region since one month. The patient gave history of trauma to the same region about 8 years back but no treatment was taken. On intraoral examination, maxillary left lateral incisor had discoloration and was tender on percussion. Also, maxillary left first molar was found to be grossly carious. The patient was advised for intraoral periapical (IOPA) radiographs for both the teeth. IOPA radiograph for maxillary left lateral incisor (Fig. 1) revealed radiolucency with respect to apical third of the root. IOPA radiograph with respect to maxillary left first molar (Fig. 2) showed furcation involvement with small radiolucency in relation to mesiobuccal root apex. A provisional diagnosis of periapical cyst was given. The treatment plan for maxillary left lateral incisor was root canal therapy with apicoectomy of the cystic lesion and extraction of left maxillary first molar along with periapical curettage. The cystic lesion obtained following apicoectomy as well as the extracted maxillary left first molar with soft tissue attached to its mesiobuccal root was sent to the Deptt of Oral Pathology & Microbiology for histopathological examination.
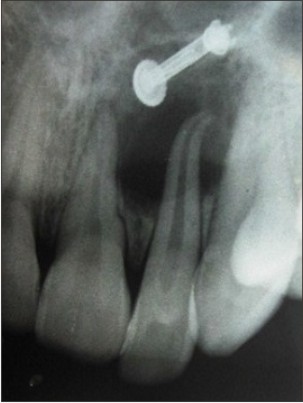 | Fig 1. IOPA radiograph of maxillary left lateral incisor showing radiolucency at the root apex.
 |
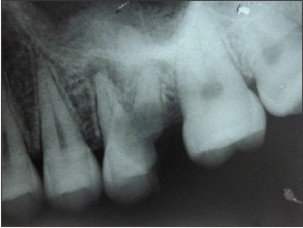 | Fig 2. IOPA radiograph of maxillary left first molar showing radiolucency at the mesiobuccal root apex.
 |
Both the soft tissue specimens were fixed in 10% buffered formalin and processed using routine manual processing technique. After paraffin embedding, 4μ thin sections were cut from each specimen and stained with routine manual H & E technique.
The H & E stained section from the soft tissue associated with maxillary left lateral incisor (Fig. 3a, 3b) showed non keratinized stratified squamous epithelium supported by a connective tissue wall. Arcading pattern was seen in the epithelium. Many eosinophilic, linear and curved rushton bodies were seen throughout the lining epithelium. The connective tissue wall was densely infiltrated with chronic inflammatory cells. Areas of extravasated red blood cells were seen. The features were suggestive of radicular cyst and hence the diagnosis.
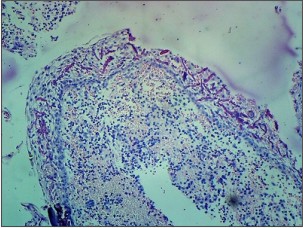 | Fig 3. Photomicrograph of radicular cyst in relation to maxillary left lateral incisor showing rushton bodies (arrow), (a) H&E, 10x.
 |
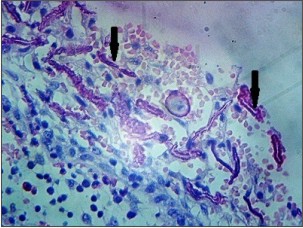 | Fig 3. Photomicrograph of radicular cyst in relation to maxillary left lateral incisor showing rushton bodies (arrow), (b) H&E, 40x
 |
The H & E stained section of the tissue from left maxillary first molar (Fig. 4a,4b) showed a cystic lumen lined by non keratinized stratified squamous epithelium which was supported by a connective tissue wall. The epithelium showed proliferation in arcading pattern. Few eosinophilic, linear and slightly curved rushton bodies were seen within the epithelial lining in one area. The connective tissue wall was moderately collagenous showing dense chronic inflammatory cell infiltration. Vascularity was moderate. The histopathological diagnosis of radicular cyst was given.
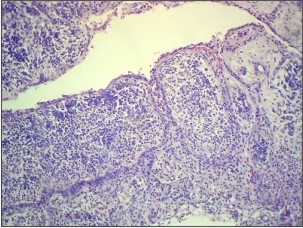 | Fig 4. Photomicrograph of radicular cyst in relation to maxillary left first molar showing rushton bodies (arrow), (a) H&E, 10x.
 |
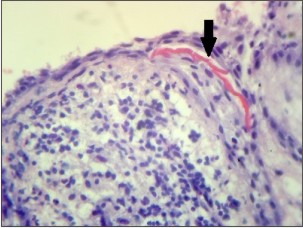 | Fig 4. Photomicrograph of radicular cyst in relation to maxillary left first molar showing rushton bodies (arrow), (b) H&E, 40x
 |
Discussion
The epithelial linings of the radicular cysts are derived from the epithelial cell rests of Malassez in the periodontal ligament which come to lie in periapical granulomas associated with teeth with necrotic, often infected, pulps. Thus, the epithelial cell rests are initiated to proliferate by inflammation as a result of necrotic debris and bacterial antigens derived from the dead pulp.[3] A cyst cavity forms within a proliferating epithelial mass in an apical granuloma by degeneration and death of cells in the centre.
The epithelial linings may be discontinuous in part and range 6–20 cell layers thick in majority of cases. In early cysts, the epithelial lining may be proliferative and show arcading with an intense associated inflammatory process but as the cyst enlarges the lining becomes quiescent and fairly regular with a certain degree of differentiation to resemble a simple stratified squamous epithelium. Keratin formation (mainly orthokeratinisation) may be seen affecting part of the lining epithelium and is seen in about 2% of radicular cysts.[1]
Hyaline bodies were first described by Dewey[4] in 1918 and later by Rushton[5] in 1955 in the epithelial lining of radicular cyst and hence are now referred to as Rushton bodies.
Since its discovery, there has been a continuous debate regarding the origin of Rushton bodies. Rushton[5] believed that they resembled the keratinised secondary enamel cuticle of Gottlieb in appearance and the liability to fracture. Shear[6] indicated that they were of odontogenic epithelial origin and probably a form of keratin depending upon histochemical studies and his view was supported by Takeda et al.[7]
Hematogenous origin was also proposed by few authors who suggested that these were derived from thrombi in venules of the connective tissue that had become varicose and strangled by epithelial cuffs which encircled them.[8],[9] Later, Browne and Matthews[10] stained cysts containing hyaline bodies for keratin, Factor VIII-related antigen, haemoglobin and fibrinogen, using immunoperoxidase methods. The hyaline bodies were negative for all these antigens but fibrinogen was detected in the cores of some circular and polycyclic forms. They tentatively proposed that the presence of fibrinogen in the cores of some hyaline bodies could support the notion of a haematogenous origin of the granular bodies.[10]
However, the ultrastructural studies failed to demonstrate any relation of rushton bodies either to RBCs or blood vessels. Rather it was suggested that the bodies are a secretory product of odontogenic epithelium deposited on the surface of particulate matter such as cell debris or cholesterol crystals in a manner analogous to the formation of dental cuticle on the unerupted portions of enamel surfaces.[11]
Scanning electron microscopy showed that the hyaline bodies were more or less spherical structures consisting of concentrically laminated layers which on section resembled a cut onion. The surface of each layer had a fine-grained texture.[12]
Few authors have reported the presence of rushton bodies in odontogenic keratocyst, dentigerous cyst, glandular odontogenic cyst and in ameloblastoma.[7],[13],[14] They appear to be restricted to odontogenic lesions.
Hence, although the origin of hyaline bodies remains obscure, it is generally now thought that they represent a secretory product of odontogenic epithelium.
Conclusion
Radicular cysts being the most common odontogenic inflammatory cysts are very frequently encountered in dental clinics whereas rushton bodies are seen in only 10% of radicular cysts. In our case, the patient had two radicular cysts and rushton bodies were seen in the epithelial lining of both the cysts which is unique.
Their presence in few other oral lesions has been reported but it is limited to lesions of odontogenic origin. These are now believed to be secretory products of odontogenic epithelium.
Acknowledgement
We thank Mr. Ranvir Singh for his technical assistance.
References
1. Shear M, Speight P. Cysts of Oral and Maxillofacial Regions, 4th edition. Copenhagen: Blackwell Munksgaard;2007.
2. Jones AV, Craig GT, Franklin CD. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J Oral Pathol Med 2006;35:500–507.
3. Meghji S, Qureshi W, Henderson B, Harris M. The role of endotoxin and cytokines in the pathogenesis of odontogenic cysts. Arch Oral Biology 1996;41: 523–531.
4. Dewey KW. Cysts of the dental system. Dent Cosmos 1918;60:555–570.
5. Rushton MA. Hyaline bodies in the epithelium of dental cysts. Proc Royal Soc Med 1955;48:407–409.
6. Shear M. The hyaline and granular bodies in dental cysts. B Dent J 1961;110:301–307.
7. Takeda Y, Kikuchi H, Suzuki A. Hyaline bodies in ameloblastoma: histological and ultrastructural observations. J Oral Pathol 1985;14:639–643.
8. Bouyssou M, Guilhem A. Recherches morphologiques et histochimiques sur les corps hyalins intrakystiques de Rushton. Bulletin du Groupement International pour la Recherche Scientifique en Stomatologie et Odontologie (Bruxelles) 1965;8:81–104.
9. Sedano HO, Gorlin RJ. Hyaline bodies of Rushton; some histochemical considerations concerning their etiology. Oral Surg Oral Med Oral Pathol 1968;26:198–201.
10. Browne RM, Matthews JB. Intra-epithelial hyaline bodies in odontogenic cysts: an immunoperoxidase study. J Oral Pathol 1985;14:422–428.
11. Morgan PR, Johnson NW. Histological, histochemical and ultrastructural studies on the nature of hyaline bodies in odontogenic cysts. J Oral Pathol 1974;3:127–147.
12. Philippou S, Rühl GH, Mandelartz E. Scanning electron microscopic studies and X-ray microanalysis of hyaline bodies in odontogenic cysts. J Oral Pathol Med 1990;19:447–452.
13. Lam KY, Chan AC. Odontogenic keratocysts: a clinicopathological study in Hong Kong Chinese. Laryng 2000;110:1328–1332.
14. Ide F, Shimoyama T, Horie N. Glandular odontogenic cyst with hyaline bodies: an unusual dentigerous presentation. J Oral Pathol Med 1996;25:401–404.
|