Introduction
Oral cavity is colonized by a variety of microorganisms. When these microorganisms adhere to the surface of tooth and grow in the prevailing environment they form bacterial biofilms which are considered to be the primary etiological factor for the initiation and progression of periodontal disease.[1],[2]
Dental calculus is formed by the calcification of plaque.[3] Plaque is composed of organic, inorganic materials derived from saliva, gingival crevicular fluid and bacterial products. Since calculus is a calcified deposit, it has been stated that dental calculus itself is not harmful, its presence in association with dental plaque may influence the severity and progression of periodontal disease.[4] Mineralization of calculus has been shown to be highly variable, containing a variety of crystalline forms.[5] In heavy calculus formers, 40-65 % mineralization can be found in plaque formed during a 3-14 day period while mature calculus may contain up to 80 % mineral and 20 % of organic matrix.[3] It has been demonstrated that the environment within the biofilms is able to support viable bacterial communities through molecular diffusion of nutrients through channels. Possibility therefore exists that a favorable environment for oral bacteria may be found within the dental calculus and that such bacteria may remain viable for some time making calculus a possible source of infection.[6] Various techniques have been used to demonstrate the viability of bacteria including bacterial culture, fluorescent microscopy and confocal laser scanning microscopy.[4] The aim of the present study, thus, was to investigate the viability of bacteria within dental calculus using fluorescent and dark field microscopy.
Material and Methods
30 subjects in the age group of 35-50 years with clinical evidence of chronic inflammatory periodontal disease were selected. Supra and subgingival calculus were harvested from these subjects. Patients with the history of any systemic disease or having undergone any antimicrobial therapy for at least past six months; oral prophylaxis for at least six months prior to harvesting the sample; pregnant, lactating women; and those individual with salivary gland disease and xerostomia were excluded from the study.
Care was taken to obtain large single pieces and to maintain the integrity of the calculus sample. Harvested sample was placed in sterile saline. The calculus sample was then crushed aseptically between two sterile slides and was then divided into three groups for gram staining, fluorescent and dark field microscopy.
Gram staining: One part of crushed sample was stained with gram stain and viewed using bright field microscopy at 100× oil objective to view the presence of gram positive and gram negative bacteria.
Fluorescent microscopy: To the second part of sample, 0.01% acridine orange stain was added and mixed gently with a sterile loop on a slide. A cover slip was placed over the mix. The stained sample was examined using fluorescent microscopy at 40× objective with filter 3A 380-460nm and then at 100x.
Dark field microscopy: The third part of crushed sample was mixed with a drop of saline and cover slipped. The preparation was observed using dark field microscopy at 40x objective and then at 100x.
Nikon Eclipse 80i research microscope was used for all three types of microscopy.
Results
Gram staining was used to visualize and determine the morphology of bacteria. Gram-negative bacteria formed 40% of all microflora in the form of rod shape bacilli and gram-negative cocci formed 30%. The remaining 30% include filamentous microorganisms occur singly or in groups within the sample (Fig. 1). No gram-positve micro organisms were seen. Using acridine orange fluorescent stain viable bacteria showed green fluorescence while non-viable bacteria appeared red (Fig. 2). The viable bacteria formed 40% of all microorganisms in the sample. Dark field microscopy examination revealed the presence of motile filamentous organisms, spirochetes and motile short bacilli indicating the viability of these microorganisms (Fig. 3).
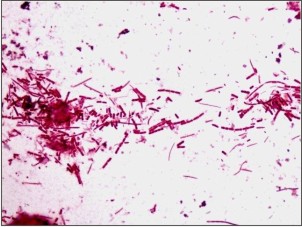 | Fig.1: Gram staining showing Gram negative cocci, bacilli and filamentous bacteria
 |
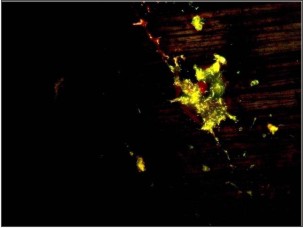 | Fig.2: Acridine orange staining showing green colored appearance of bacteria
 |
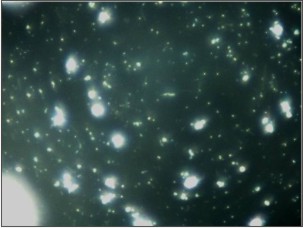 | Fig.3: Dark field microscopic showing presence of motile spirochetes and filamentous organisms.
 |
Discussion
Micro radiographic and electron microscopic studies of formed calculus have shown that variety of morphologically different micro organisms both gram-positive and gram-negative species become calcified when immersed in a calcium phosphate solution.[7] Although there are reports in the literature implying that calculus may be essentially mineralized dead organic material, it has been shown that calcifications can occur in a culture of live bacterial colonies. Therefore, it is possible that some microorganisms may readily calcify while other may not, leading to the creation of pockets of non-mineralized bacteria within calcified plaque sample.[8] Thus, calculus may act as a major source of infection in the oral cavity. Few techniques have been used in previous studies to distinguish between viable and nonviable bacteria in biofilms.[4] Gram stain is used mainly to visualize bacteria and also study the morphological characteristics of all microorganisms.[9] In present study no gram-positive organisms were seen, only gram-negative bacilli, cocci and filamentous organisms were noticed.
Acridine orange, a fluorochrome stain, is potentially superior to the Gram stain in the direct microscopic examination of clinical specimens because it a gives striking differential staining between bacteria and background cells and debris. Lauer assessed its value in clinical laboratories by testing 209 cerebrospinal fluids and 288 other body fluids, tissues, and exudates using gram stain and acridine orange staining techniques. Overall, acridine orange was found to be slightly more sensitive than the gram stain with acridine orange showing 59.9% sensitivity and gram stain showing 55.8% but both the stains showed a similar specificity in detecting microorganisms.[10] In the present study, acridine orange staining technique revealed viable green fluorescing bacteria which formed 40% of the total microflora of the sample. Nonviable bacteria were stained red. Acridine orange stain has marked affinity for the nucleic acids and stains both viable and non-viable bacteria. When the sample were stained DNA component of the organisms appeared green.[2] As acridine orange stain is applied to calculus sample, it binds with the nucleic acid component, DNA, of the bacteria. On fluorescent microscopy acridine orange-DNA complex absorbs incoming radiation and affects the wavelength of emitted radiation, because of the ring structure of acridine orange and the abnormal chemistry of these complexes. This makes the acridine orange-DNA complex to show green fluorescence in viable bacteria.[10] As viable bacteria become non-viable, DNA gets denatured resulting in increased acridine orange inter-chelating with the phosphate-sugar backbone of DNA.[11] This lead to change in fluorescence form green to red in non-viable bacteria.[12],[13]
Earlier studies have tried to demonstrate bacterial viability within the calculus sample using bacterial cultures.[2] Sidaway DA in (1978)[14] reported successful bacterial cultures from the supragingival and subgingival calculus sample but also found that some microorganisms are difficult to culture but can be easily accessed by dark field microscopy as it shows the presence and motility of filamentous organisms, spirochetes and short bacilli thus confirming their viability. Presence of motile spirochete, filaments and other bacilli in dark field microscope in the present study also indicates that some of bacteria in the calculus remain viable.
Conclusion
This study provides strong evidence indicating that viable micro organisms are contained within the calculus probably within the lacunae and channels. Vital bacteria in calculus may release toxic antigenic metabolites, which may leach out of this calcified mass and initiate inflammatory responses into the soft tissues. Thus, calculus may serve as a reservoir of viable micro organisms and play a crucial role in the etiology and recurrence of oral infections even after treatment.
References
1. Nagayoshi M, Fukuizumi T, Kitamura C, Yano J, Terashita M, Nishihara T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol 2004: 19:240–246.
2. Moolya NN, Thakur S, Ravindera S, Setty SB, Kulkarni R, vibility of bacteria in dental calculus – a microbiological study. J Indian soc of Periodontol 2010;14:222-226.
3. Sidway DA. A microbiological study of dental calculus. A comparison of the in vitro calcification of viable and non-viable microorganism. Periodontal Res 1979;14:167-172.
4. Tan B, Mordan NJ, Embleton J, Pratten J, Galgut PN. Study of bacterial viability within human Supragingival dental calculus. J Periodontol. 2004;75:23-29.
5. Tan B, Gillam DG, Mordan NJ, Galgut PN. A preliminary investigation into the ultrastructure of dental calculus and associated bacteria. J Clin Periodontol 2004;31:364–369.
6. Bauhammers H, Rohrbaugh EA. Permeability of human and rat dental calculus. J Periodontol 1970;41:39-42.
7. Selvig KA. The formation of plaque and calculus on recently exposed tooth surfaces. Periodontal Res 1969;4:10-11.
8. Wasserman BH, Mandel ID, Levy BM. In vitro calcification of dental calculus. J Periodontol 1958:29;144-147.
9. Winn W, Allen S, Janda W, Koneman E , Procop G, Schreckenberger P. Koneman’s colour atlas and text book of diagnostic microbiology. 6th ed. USA: Lippincott Williams and Wilkins; 2006. p. 2-64.
10. Lauer BA, Reller LB, Mirrett S. Comparison of acridine orange and Gram stains for detection of microorganisms in cerebrospinal fluid and other clinical specimens. J Clin Microbiol 1981;14:201-5.
11. Miliotis MD. Acridine orange stain for determining intracellular enteropathogen in HeLa cells. J clin microbiol1991;29:830-831.
12. Goldner M, Himsley HF, Kormendy. A Bacterial phagocytosis monitored by fluorescence and extracellular quenching: ingestion and intracellular killing. Lab. Med. 1983;14:291-294.
13. Pruzanski W, Saito S, Nitzan DW. The influence of lysostaphin on phagocytosis, intracellular bactericidal activity, and chemotaxis of human polymorphonuclear cells. J Lab Clin Med. 1983;102:298–305.
14. Sidaway DA. A microbiological study of dental calculus. The microbiological flora of mature calculus. J Period Res 1978; 13:349-59.
|