Introduction
Halitosis is a medical term, first coined by the Listerine Company in 1921, used to describe unpleasant breath, regardless of its sources, oral or non-oral. The word originates from the Latin “halitus” meaning “breath” and the Greek “osis” meaning “abnormal” or “diseased. Halitosis is not a disease but rather a symptom of underlying oral, systemic or psychological conditions. The primary cause of halitosis is due to the release of odoriferous volatile sulphur compounds (VSC) in the exhaled air.[1] Most frequent sources of halitosis (80–90%) exist within the oral cavity and include bacterial reservoirs such as the dorsum of the tongue, saliva and periodontal pockets, where anaerobic bacteria degrade sulphur-containing amino acids to produce the foul smelling volatile sulfur compounds.[2] Oral malodor also can originate from non-oral causes, which include diabetic ketosis and acidosis; uremia; erratic bowel movement and regurgitations; hepatic and renal failure.[3] The three primary measurement methods of halitosis are organoleptic measurement, gas chromatography, sulphide monitoring.[1] Before treating oral malodor the dental practitioner should assess all oral diseases and conditions that may contribute to oral malodor. Therefore, more than any other health professional, dentists ought to be well informed on halitosis in order to provide effective treatment and proper advice to the significant proportion of the general population affected by this condition.
Measurement Methods Of Halitosis:
The three primary measurement methods of genuine halitosis are:
1. Organoleptic measurement[4],
2. Gas chromatography[5], and
3. Sulphide monitoring.[6],[7],[8]
Additional or alternative measurement methods are:
4. BANA test[9],
5. Chemical sensors,[10]
6. Quantifying β-galactosidase activity[11],
7. Salivary incubation test,[12]
8. Ammonia monitoring,[13]
9. Ninhydrin method,[14]
10. Polymerase chain reaction,[15]
11. Tongue Sulfide Probe,[16]
12. Zinc Oxide Thin Film Conductor Sensor,[17]
13. OraTest[18], and
14. Self Assessment of Oral Malodor[19]
1) Organoleptic measurement
Organoleptic or hedonic measurement is a simple commonly used measurement method. A plastic tube is inserted into the patient’s mouth, preventing the dilution of mouth air with room air. While the patient is exhaling slowly, the examiner judges the odour at the other end of the tube. Before acting as a judge, persons must ensure that they do not have anosmia (lost or impaired smelling capacity). Odor judges should test their capacity to smell and recognize different odors (qualitative assessment) as well as their capacity to detect odors at low concentrations (quantitative assessment).[20] They are also required to refrain from drinking coffee, tea or juice, and to refrain from smoking and using scented cosmetics before the assessment.[21] For assessment patients are instructed to abstain from taking antibiotics for three weeks before the assessment, to abstain from eating garlic, onion and spicy foods for 48 hours before the assessment and to avoid using scented cosmetics for 24 hours before the assessment. Patients are instructed to abstain from ingesting any food or drink, to omit their usual oral hygiene practices, to abstain from using oral rinse and breath freshners, and to abstain from smoking for 12 hours before the assessment. [4]
A plastic tube is inserted into the patient’s mouth, preventing the dilution of mouth air with room air. While the patient is exhaling slowly, the examiner judges the odour at the other end of the tube. A privacy screen with a hole for the straw or the tube can be used to separate the examiner from the patient.
Various scoring systems[22],[23] can be used for estimating the intensity of the odour. The most widely used scale is described by Rosenberg et al[22]:
Grade 0 no appreciable odor.
Grade 1 barely noticeable odor.
Grade 2 slight but clearly noticeable odor.
Grade 3 moderate odor.
Grade 4 strong odor.
Grade 5 extremely foul odor.
Although a good correlation between VSC’s concentration and organoleptic values has been found, it is still a subjective test, and when the examiners are repeatedly exposed to bad odors they become adapted to them and lose sensitivity. Dentists, especially periodontists, may not be ideal judges if they do not use masks on a regular basis. There is also the potential risk of disease transmission to the examiner through the expelled air. This is particularly important with the existence of epidemic bird flu infections and other acute respiratory illnesses.[3] A possible solution to overcome these limitations is to use a more systemized and standardized method of organoleptic testing for measuring halitosis.
One of the most standardized method is Kim organoleptic method,[24] which uses a gastight syringe and a paper cup connected to a plastic straw to measure halitosis. The advantages of this method are that the subject is prevented from observing the direct sniffing procedure of the examiner, the oral air samples can be separated from the subjects, and low concentrations of gases can be detected. It is also possible to obtain halitosis samples from the peri-oral region that are not diluted by room air.
The Spoon test[25] can also be used for organoleptic measurement. It is a simple, albeit subjective measurement method. Using a spoon or similar instrument, the tongue dorsum is scraped and the scraped material can be smelled.
2) Gas chromatography
Quantitative analysis of VSC’s by a gas chromatography (GC) equipped with a flame photometric detector (FPD) is considered one of the most reliable measurements for diagnosing halitosis. The concentration of VSC’S in samples of saliva, tongue coating or expired breath is measured by producing mass spectra. The components can be identified by comparing the mass spectra with those of a computer based reference library. Gas chromatography may be combined with mass spectrometry, enlarging the scope of the method.[5]
GC-FPD measurement is very dependable because of its specificity to VSC’s and is highly objective and reproducible. The disadvantage of using a GC- FPD is that the equipment being sophisticated, requires an experienced operator and the device is costly and very large. Therefore, it is impractical to use it for routine examinations in dental practices.[26]
A compact and simple Gas Chromatography equipped with a newly invented indium oxide (In2O3) semiconductor gas sensor (SCS), [26] which is highly sensitive to all kinds of VSC’s has been developed recently. GC-SCS measures each VSC’s separately, whereas other devices cannot detect each separately.
The cost of GC-SCS is 15% to 25% of a conventional GC-FPD.[26] Also, it does not require hydrogen and carrier gas, which are essential for GC-FPD. The method is considered to be highly objective, reproducible, and reliable, but still it cannot be easily clinically implemented because of the relatively high cost, the requirement of highly trained persons, and the extensive procedures.
In order to overcome the practical drawbacks, portable gas chromatographs were developed to measure sulphur-containing compound levels inside the mouth and to eliminate discrepancies caused by variations in operator sampling or breath injection techniques. [26]
3) Sulphide monitoring
Portable VSC detectors, such as a sulfide monitor, are widely used for the quantitative measurement of oral malodor.
Patients are asked to refrain from talking 5min prior to measurement. The monitor is zeroed on ambient air. Measurement is performed by inserting a disposable tube into the patient’s mouth and connecting this to the monitor, while the patient is breathing through the nose. Electrochemical reactions with the sulphur-containing compounds in the breath generate an electric current, which is directly proportional to the levels of volatile sulphur-containing compounds.
Trade Names of portable sulfide monitors:
I) Halimeter® (Rosenberg et al.)[6]
II) Oral Chroma™ (Miyazaki)[7]
III)Breathtron ® (Sopapornamorn)[8]
I) Halimeter® (Interscan , US) (Figure 1)[6]
The Halimeter® device detects the presence of VSC’s that are known to produce undesirable odours. Several readings are taken from areas such as the front of the mouth, the back of the mouth and the nostrils.
The Halimeter® needs to be calibrated to zero on ambient air prior to each measurement. First, the disposable straw, connected to the Halimeter®, is placed in the opening of the nose of the patient. Then the patient is asked to blow slowly through to the nose. The maximum peak value of VSCs is recorded. Second, the patient is asked to close the mouth for 1 minute. Then the patient is asked to open the mouth and protrude the tongue. The straw is placed at the dorsal posterior mid part of the tongue and fixed until again the maximum peak value of VSCs is recorded. Peak VSC levels are registered in parts per billion (ppb)
The Halimeter® is unsuitable for measuring patients with extraoral halitosis from dimethyl sulfide.[6] The Halimeter® has a high sensitivity for hydrogen sulfide but a lower sensitivity for methyl mercaptan, which is a significant contributor to halitosis. Certain foods such as garlic and onions produce sulfur in the breath for as long as 48 hours and may result in false readings.[27] The Halimeter® is also very sensitive to alcohol, so one should avoid drinking alcohol or using alcohol containing mouthwashes for at least 12 hours prior to being tested.[28]
II) Oral Chroma™ (Abimedical Corporation) (Figure 2)[7]
Oral Chroma™ analyses the VSC’s, measures the individual concentrations of hydrogen sulfide, methyl mercaptan and dimethyl sulfide, and displays the concentrations on a display panel. Each of the three component gases, and the measured concentrations, can be correlated with a specific cause of halitosis.[7]
III) Breathtron® (Yoshida, Tokyo, Japan) (Figure 3)[8]
It is a semiconductor type sulfide monitor, which is composed of an air intake, sensor detector, control panel, digital display and printer. The semi-conductor sensor is based on a thick Zinc Oxide (ZnO) membrane that has a high specificity for VSC’s.[8]
The disposable mouthpiece, which has a build-in filter to eliminate other volatile compounds (like ketone and alcohol in toothpaste and mouth wash), is inserted into an end of the Teflon tube connected to the monitor inlet. Measurements are performed by directly inserting the disposable mouthpiece into the patient’s oral cavity. The patients are instructed to close their mouth tightly and breathe through their nose during the measurement. The aspiration rate of mouth air is 40–60 ml per minute, and the Breathtron® values are presented in units of parts per billion (ppb).[29]
Because of this monitor’s problem with differentiating sulfide compounds and because methyl mercaptan is three times more unpleasant than hydrogen sulfide at the same concentration, it is possible that Breathtron® underestimates the malodor in people with high methyl mercaptan concentrations in their mouths.[30]
Sulphide monitor measurements correlated significantly for mainly low correlation coefficients with organoleptic scores.[22],[31],[32] Patients may produce normal sulphide monitor measurements, whereas organoleptic scores are high. The reason for this discrepancy is that in addition to volatile sulphide-containing compounds other odorants contribute to halitosis, such as volatile short-chain fatty acids, polyamines, alcohols, phenyl compounds, alkanes, ketones, and nitrogen-containing compounds. These odorants are not detectable by a sulphide monitor.
Although several studies demonstrated that gas chromatography and sulphide monitor measurements are highly significant correlated, appreciable differences were observed.[20],[33],[34] The sensitivity and specificity of gas chromatography (0.79 and 0.83, respectively) appeared higher than the sensitivity and specificity of sulphide monitoring (0.76 and 0.78, respectively).[20] When relatively precise measurements are required, gas chromatography is the preferable method.[33]
Recently, a new sulphide monitor was developed. The monitor’s sensitivity and specificity was, respectively, more than 0.79 and between 0.61 and 0.73. Because this monitor has a low specificity in periodontal disease patients, it should be used cautiously for measuring volatile sulphur-containing compounds related to periodontal disease.[34]
4) BANA (benzoyl-DL-arginine-a-naphthylamide) test
It is a chairside test that is used to determine the proteolytic activity of certain oral anaerobes that contribute to oral malodor. Proteolytic obligate Gram-negative anaerobes and short-chain fatty acids colonizing the subgingival plaque and the dorsum of the tongue can be detected by the presence of an enzyme degrading benzoyl-DL-arginine-a-naphthylamide (BANA), a synthetic trypsin substrate, and forming a colored compound.[9]
Using a step-wise multiple regression analysis technique that combines a positive BANA test with Halimeter® readings vastly improves the correlation of the combined readings with organoleptic scores. BANA scores correlated significantly with organoleptic measurements, but were poorly related to sulphide monitor measurements. Probably, micro-organisms associated with BANA assay contribute malodorous components to the breath air other than sulphur-containing compounds, such as cadaverine.[35]
5) Chemical Sensor
Chemical sensors for volatile sulphur-containing compounds have been integrated into a probe for measuring directly in periodontal pockets and on the tongue. A sulphide-sensing element in the probe generates an electrochemical voltage proportional to the concentration of sulphide ions present.[10] This voltage is measured relative to the operating point of a reference element. The electrochemical voltages generated by sulphide ions are measured by an electronic unit and displayed in a digital score.
The Electronic Nose technique has recently been introduced, but the equipment is extremely costly. It can also detect organic compounds, aromatic compounds, amine-containing compounds, and ammonia derivatives in food and beverages. High correlations of this method have been found with organoleptic as well as gas chromatography measurements.[10],[36]
A traditional problem with the use of electronic noses is the influence of water vapor. This technique cannot determine volatile chemicals precisely, and it is difficult to distinguish mouth-air compounds from others present using this equipment. The mouth-air sample will be contaminated with a certain amount of respiratory air by this sampling procedure.[36]
Other promising chemical sensors for measuring ammonia and methyl mercaptan in breath air have been introduced lately.[37]
6) Quantifying β-galactosidase activity
Deglycosylation of glycoproteins is considered as an initial step in oral malodour production. β -Galactosidase is one of the important enzymes in deglycosylation. The activity of β galactosidase can be easily quantified with the use of a chromogenic substrate absorbed onto a chromatography paper disc. Saliva applied to the paper disc, may induce a colour change of the paper, which can be recorded by an examiner.[11]
7) Salivary incubation test
Saliva is believed to be one of the main sources of oral malodor because it contains a large reservoir of sulphur-containing substrates that can be hydrolyzed and further degraded to VSC. Therefore, salivary samples may be used for an indirect malodor examination.
The salivary incubation test uses saliva collected in a glass tube. After incubating the tube at 37.8oC in an aerobic chamber under an atmosphere of 80% nitrogen, 10% carbon dioxide, and 10% hydrogen for several hours, the odour can be measured by an examiner.
The salivary incubation test is much less influenced by external parameters, such as subjectivity, smoking, drinking coffee, eating garlic, onion, spicy food, and scented cosmetics, than organoleptic measurements.
In a pilot study for the evaluation of oral malodor by an in vitro salivary incubation test a strong correlation between the salivary incubation test and organoleptic as well as sulphide monitor measurements was demonstrated.[12]
8) Ammonia monitoring
A portable monitor for measuring ammonia has been developed on the basis of the hypothesis that ammonia produced by oral bacteria reflects halitosis. Bacteria in dental plaque and tongue coating produced ammonia in a concentration dependent manner. The ammonia level decreased after the removal of tongue coating and dental plaque.
Patients are instructed to rinse with a urea solution for 30 seconds and to then keep their mouth closed for 5 min. The instrument contains a pump, which can draw air through an ammonia gas detector tube connected to a disposable mouthpiece placed inside a patient’s mouth. The concentrations of ammonia produced by oral bacteria can be read directly from a scale.[13]
9) Ninhydrin Method
Amines or polyamines cannot be measured by using sulphide monitoring. The Ninhydrin colorimetric reaction is a simple, rapid, and inexpensive method. In a recent study, the ninhydrin method was used for detecting low-molecular-weight amines in breath.[14]
A sample of saliva and isopropanol is mixed and centrifuged. The supernatant is diluted with isopropanol, buffer solution (pH 5), and ninhydrin reagent. The mixture is refluxed in a water bath for thirty min, cooled to 21.8 oC, and diluted with isopropanol to a total volume of 10ml. Light absorbance readings are determined using a spectrometer.
10) Polymerase chain reaction
Real-time polymerase chain reaction (PCR) using the TaqMan system can be used for quantitative analysis of volatile sulphur-containing compounds-producing oral bacteria (e.g. Tannerella forsythensis)[15]
11) Tongue Sulfide Probe
Sensors for VSC’s have been integrated into periodontal probes and paddles which can be placed directly into the pocket or on to the tongue, again yielding significant co-relation with organoleptic scores. The tongue sulfide probe size is 0.25 x 0.75 inches and was developed to determine the sulfide levels on the tongue dorsum.[16] It is composed of an active sulfide sensing element and a stable sulfide element. The tongue sulfide probe is applied on the anterior, middle or the posterior part of the tongue along the median groove of the tongue dorsum with a light pressure for 30 sec. The sulfide-sensing element generates an electrochemical voltage proportional to the concentration of sulfide ions present. The voltage is measured relative to the operating point of reference element. The electrochemical voltage generated by the sulfide is measured by the electronic unit, and are displayed in a digital score ranging from 0.0 (undetectable sulfide levels: less than 10-7 of sulfide) to 0.5 (more than equal to 10-2 M of sulfide) in increments of 0.5.[16]
12) Zinc Oxide Thin Film Conductor Sensor
For measuring trace volatile sulfur compounds in mouth air a zinc-oxide thin film semiconductor sensor was developed. The results obtained by this device co-related with the values of total VSC’s measured by gas chromatography and also with organoleptic scores given by the judges.[17]
Since this monitor does not discriminate between gases, but presents the measure as a total gas mixture, the possible influence of acetone on the value of VSC monitor was solved with the use of a filter, which removed acetone and other compounds, such as acetaldehyde and ethyl alcohol, which are often present in mouth air.[17]
13) OraTest
The test provides quantitative assessment of the level of microbial activity in the oral cavity. The test involves oral rinsing with a sterile milk sample, followed by expectoration into a test tube containing a oxidation-reduction reduction indicator (methylene blue). The higher level of micro-organisms, the faster the color changes from blue (aerobic condition) to white (anaerobic condition) at the bottom of the test tube. In addition to co-relation with microbial counts, the OraTest exhibits significant co-relation with plaque and gingival indices.[18]
14) Self assessment of oral malodor
The age-old method of breathing into the cupped palms to discern one’s own breath may or may not detect anything and also rating of severity and improvement / degradation over time is not possible. The value in self-diagnosing may be in establishing a suspicion of a problem.[19]
Different methods used are-
A) By licking the wrist with the length of the tongue (including as far back as possible) and waiting 5 seconds before sniff-testing, one is allegedly able to discern negligible or problematic tongue odors.
B) By doing the same with floss, one can detect negligible or problematic periodontal odors.
C) By having another person evaluate mouth breath (while nose pinched closed) versus nose expirations (while holding mouth closed) can help detect odors of sinus origin.
D) Subjects are asked to score their own oral malodor on a continuous 10-cm visual analogue scale (VAS) marked on each end as "no odor" and "extremely foul odor", respectively.
Summary
Halitosis or breath malodour may be an indicator for a medical problem and in many cases may cause significant social problems. In most of cases, halitosis is of oral origin. A clinical diagnosis of oral malodour ultimately is a subjective judgement. A number of different methods have been described for clinical assessment of oral malodour. But measurement of halitosis is complicated by a variety of parameters and each method has specific advantages and shortcomings with respect to these parameters. Self assessment of oral malodour is notoriously unreliable. Nevertheless, the use of organoleptic measurement is suggested as the gold standard or primary indicator of halitosis. Although this method clearly has a degree of subjectivity, the necessary training aims to make the assessment as objective and reproducible as possible both within and between examiners, although the reliability and reproducibility of the method has been questioned. Gas chromatography is the preferable method if precise measurement of gases are required, but the method cannot be easily clinically implemented since it requires relatively high cost, highly trained persons, and extensive procedures. Sulphide monitoring is a relatively inexpensive and easily used methods, Levels of volatile sulphur compounds as measured by Halimeter® are reported to correlate well with organoleptic assessments, although it should be borne in mind that other compounds in addition to volatile sulphur compounds may contribute to oral malodour and these compounds would not be detected with a volatile sulphur compounds meter. The scientific and practical value of the additional or alternative measurement methods of halitosis presented in this review is still to be established by scientific evaluation of methods. The most promising additional or alternative method for both research and clinical purposes seem to be the use of chemical sensors.
Thus, the dentist, and in particular the periodontologist, should be able to diagnose it and should be able to offer adequate treatment.
 | Figure 1 Halimeter (Interscan Corporation)
 |
 | Figure 2 Oral Chroma (Abimedical Corporation)
 |
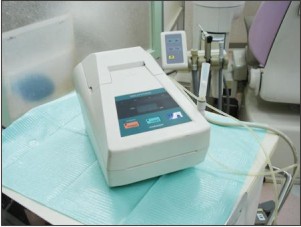 | Figure 3 Breathtron (Yoshida Corporation)
 |
Refernces
1. Tonzetich J. Production and origin of oral malodor: a review of mechanisms and methods of analysis. J Periodontol. 1977;48(1):13-20.
2. Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992;27(4 Pt 1):233-8.
3. Preti G, Clark L, Cowart BJ, Feldman RS, Lowry LD, Weber E, et al. Non-oral etiologies of oral malodor and altered chemosensation. J Periodontol. 1992;63(9):790-96.
4. Yaegaki K, Coil JM. Examination, Classification, and Treatment of Halitosis; Clinical Perspectives. J Can Dent Assoc. 2000;66(5):257-61.
5. Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol. 1971;16(6):587-97.
6. Halimeter: used in the diagnosis and treatment of chronic halitosis [Internet]. Chatsworth(CA): Interscan Corp.; [cited 2012 Aug 21]. Available from: http://www.halimeter.com/.
7. OralChroma: A Halitosis Measuring Device [Internet]. Osaka(JP): Abimedical Corp.; c2008 [cited 2012 Aug 21]. Available from: http://www.abilit-medical-and-environmental.jp/en/medical/index.html
8. Ueno M, Shinada K, Yanagisawa T, Mori C, Yokoyama S, Furukawa S, et al. Clinical Oral Malodor Measurement with a Portable Sulfide Monitor. Oral Dis. 2008 Apr;14(3):264-9.
9. Kozlovsky A, Gordon D, Gelernter I, Loesche WJ, Rosenberg M. Correlation between the BANA test and oral malodor parameters. J Dent Res. 1994;73(5):1036-42.
10. Tanaka M, Anguri H, Nonaka A, Kataoka K, Nagata H, Kita J, et al. Clinical assessment of oral malodor by the electronic nose system. J Dent Res. 2004;83:317–21.
11. Sterer N, Rosenberg M. Effect of deglycosylation of salivary glycoproteins on oral malodour production. Int Dent J. 2002;52 Suppl 3:229-32.
12. Quirynen M, Zhao H, Avondtroodt P, Soers C, Pauwels M, Coucke W, et al. A salivary incubation test for evaluation of oral malodor: a pilot study. J Periodontol. 2003;74(7):937-44.
13. Amano A, Yoshida Y, Oho T, Koga T. Monitoring ammonia to assess halitosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(6):692-6.
14. Iwanicka-Grzegorek K, Lipkowska E, Kepa J, Michalik J, Wierzbicka M. Comparison of ninhydrin method of detecting amine compounds with other methods of halitosis detection. Oral Dis. 2005;11 Suppl 1:37-9.
15. Suzuki N, Yoshida A, Nakano Y. Quantitative analysis of multi-species oral biofilms by TaqMan real-time PCR. Clin Med Res. 2005; 3(3): 176–185.
16. Morita M, Musinski DL, Wang HL. Assessment of Newly Developed Tongue Sulfide Probe for Detecting Oral Malodor. J Clin Periodontol. 2001;28(5):494-6.
17. Shimura M, Yasuno Y, Iwakura M, Shimada Y, Sakai S, Suzuki K, et al. A New Monitor with a Zinc-Oxide Thin Film Semiconductor Sensor for the Measurement of Volatile Sulfur Compounds in Mouth Air. J Periodontol. 1996;67(4):396-402.
18. Rosenberg M, Barki M, Goldberg S. The Antimicrobial Effect of Mouthrinsing as Measured Using the "Oratest". J Dent Res. 1996;68(4):655-662(abstr 45).
19. Rosenberg M, Kozlovsky A, Gelernter I, Cherniak O, Gabbay J, Baht R, et al. Self-Estimation of Oral Malodor. J Dent Res. 1995;74(9):1577-82.
20. Oho T, Yoshida Y, Shimazaki Y, Yamashita Y, Koga T. Characteristics of Patients Complaining of Halitosis and the Usefulness of Gas Chromatography for Diagnosing Halitosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(5):531-4.
21. Nachnani S, Majerus G, Lenton P, Hodges J, Magallanes E. Effects of Training on Odor Judges Scoring Intensity. Oral Dis. 2005;11 (Suppl 1):40-4.
22. Rosenberg M, Septon I, Eli I, Bar-Ness R, Gelernter I, Brenner S, et al. Halitosis Measurement by an Industrial Sulphide Monitor. J Periodontol. 1991;62(8):487-9.
23. Bornstein MM, Stocker BL, Seemann R, Bürgin WB, Lussi A. Prevalence of Halitosis in Young Male Adults: A Study In Swiss Army Recruits Comparing Self-Reported and Clinical Data. J Periodontol. 2009;80(1):24-31.
24. Kim DJ, Lee JY, Kho HS, Chung JW, Park HK, Kim YK. A New Organoleptic Testing Method for Evaluating halitosis. J Periodontol. 2009;80(1):93-7.
25. Greenstein RB-N, Goldberg S, Marku-Cohen S, Sterer N, Rosenberg M. Reduction of oral malodor by oxidizing lozenges. J Periodontol. 1997 Dec;68(12):1176-81.
26. Murata T, Rahardjo A, Fujiyama Y, Yamaga T, Hanada M, Yaegaki K, et al. Development of a Compact and Simple Gas Chromatography for Oral Malodor Measurement. J Periodontol. 2006;77(7):1142-7.
27. Yaegaki K, Sanada K. Effects of a two-phase oil-water mouthwash on halitosis. Clin Prev Dent. 1992;14(1):5-9.
28. Miyazaki H, Sakao S, Katoh Y, Takehara T. Correlation between volatile sulphur compounds and certain oral health measurements in the general population. J Periodontol. 1995;66(8):679-84.
29. Iwakura M, Hario H, Washio J. Oral malodor measurement (Breathtron). The Nippon Dental Review 2002;62:105–108 (in Japanese).
30. Washio J, Sato T, Koseki T, Takahashi N. Hydrogen sulfide producing bacteria in tongue biofilm and their relationship with oral malodor. J Med Microbiol. 2005;54(Pt 9):889-95.
31. Rosenberg M, Kulkarni GV, Bosy A, McCulloch CAG. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J Dent Res. 1991;70:1436–40.
32. Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol. 1971;16(6):587-97.
33. Furne J, Majerus G, Lenton P, Springfield J, Levitt DG, Levitt MD. Comparison of volatile sulfur compound concentrations measured with a sulfide detector vs. gas chromatography. J Dent Res. 2002;81(2):140-3.
34. Sopapornamorn P, Ueno M, Shinada K, Vachirarojpisan T, Kawaguchi Y. Clinical application of a VSCs monitor for oral malodour assessment. Oral Health Prev Dent. 2006;4(2):91-7.
35. Goldberg S, Kozlovsky A, Gordon D, Gelernter I, Sintov A, Rosenberg M. Cadaverine as a putative component of oral malodor. J Dent Res. 1994;73:1168–72.
36. Nonaka A, Tanaka M, Anguri H, Nagata H, Kita J, Shizukuishi S. Clinical assessment of oral malodour intensity expressed as absolute value using an electronic nose. Oral Dis. 2005;11:35–6.
37. Minamide T, Mitsubayashi K, Jaffrezic-Renault N, Hibi K, Endo H, Saito H. Bioelectronic detector with monoamine oxidase for halitosis monitoring. The Analyst 2005;130:1490–4.
|