Introduction
The ultimate goal of tooth regeneration is to replace lost teeth. Stem cell-based tooth engineering is deemed as a promising approach to the making of a biological tooth or ‘bio-tooth’.
Stem cells refer to cells that regenerate via mitotic cell division and differentiation into specialized cell types. Stem cells have the remarkable potential to develop into many different cell types in the body during early life and growth. These pluripotent stem cell populations persist in multiple organs and when stimulated they proliferate and differentiate in response to local cues provided by the organs they are recruited to (fig 1).
Stem cells can be differentiated from other cell types because of unique properties of:
I. Self renewal: stem cells can renew themselves almost indefinitely. This is also known as proliferation.
II. Differentiation: stem cells have the special ability to differentiate into cells with specialized characteristics and function.
III. Unspecialized: stem cells are largely unspecialized cells which then give rise to specialized cells.
Scientists discovered ways to derive embryonic stem cells from mice embryos nearly 30 years ago, in 1981. A detailed study of the biology of mice stem cells led to the discovery, in 1998, of a method to derive stem cells from human embryos and grow the cells in the laboratory. Research on adult stem cells has led scientists to their various applications in treatment of diseases and disorders which were once considered untreatable.
Key research events in dental stem cells
1. Scientists grow teeth in lab (Dec 11, 2002)
2. Scientists discover a unique source of Postnatal Stem Cells in 'Baby' Teeth (Apr 22, 2003)
3. Dr. Songtao Shi of NIH discovers a new source of adult stem cells in children’s primary teeth. Stem(May 2003).
4. Dental researchers have been working with stem cells with the objective: “ Grow-your-own”, to replace false teeth. (May 3, 2004)
5. Human Periodontal Ligament Stem Cells isolated for the first time. (Jul 8, 2004)
6. Scientist signals for Stem Cell studies (Feb 2005)
7. Banking Baby, Wisdom Teeth for Stem Cells (June 8, 2005)
Types of Stem Cells
Based on their origin, stem cells are categorized either as embryonic stem cells (ESCs) or as postnatal stem cells; somatic stem cells or adult stem cells. Embryonic stem cells are best derived from 2-11 days old embryo called blastocyst. ESCs are considered as immortal as they can be propagated and maintained in an undifferentiated state indefinitely. The therapeutic benefit of these totipotent cells is curtailed by moral and ethical concerns as extracting stem cells from an embryo destroys the embryo itself. Owing to these shortfalls, ESCs remained only as platform for research[1]. Adult stem cells are multipotent, i.e., they are capable of differentiating into more than one cell type but not all cell types. Moreover they have plasticity ,that is, their ability to expand beyond their recognized potential irrespective of the parent cell from which they are derived. Adult stem cells can be hemopoetic stem cells (HSCs) or mesenchymal stem cells (MSCs)[2].
Tissue engineering and regenerative medicine seek to replace lost or damaged tissues due to any reason, and this needs three major ingredients which are (Fig 2):
1. Morphogenic Signals such as growth factors and differentiation factors. These factors play an important role in the multiplication and differentiation of stem cells into the specifically needed type of cells.
BMPs (bone morphogenic proteins) and cytokines play a major role in organogenesis, and with regard to dental tissues specifically, GDf-11 (growth/differentiation factor 11), which is a novel member of BMP/TGF B family is expressed in differentiating odontoblasts. It therefore plays a major role in differentiation of dental pulp stem cells into odontoblasts, a biological phenomenon, which is the corner stone of teeth tissue engineering[3].
2.Responding stem cells which are originally harvested from the patient and preserved under good conditions to maintain their special ability to differentiate into a wide range of cells.
3.Scaffold (Fig 3) of extra cellular matrix, which provides these cells with the environment and mold to grow into what we want them to become and function.Scaffold provides physical support to the cells required for regeneration of any tissue. These scaffolds should be biodegradable and rate of degradation should coincide with the rate of tissue formation. They should be porous and allow appropriate differentiation of cells without affecting their progeny. In the root canal system it is preferable to have a scaffold that promotes vascularization of the implanted cells as pulp has blood supply only from apical end. This is achieved by impregnating the scaffolds with growth factors that promote angiogenesis[4].
Regenerative dentistry and stem cells from dentofacial origin (Fig 4)[5]:
In the year 2003 Dr. Songtao Shi, a pediatric dentist, discovered baby tooth stem cells by using the deciduous teeth of his six year old daughter. He was luckily able to isolate, grow and preserve these stem cells’ regenerative ability, and he named them as SHED (Stem cells from Human Exfoliated Deciduous teeth)[6].
Stem cells have also been isolated from orofacial tissues that include adult tooth pulp tissue, deciduous tooth pulp tissue, periodontal ligament, apical papilla (SCAPs); dental follicle precursor cells (DFPCs) and buccal mucosa. Stem cells from human exfoliated deciduous teeth (SHED) are multipotent and are far more immature in the cell hierarchy than the adult pulp stem cells. Recently mesenchymal stem cells from apical papilla of incompletely developed teeth have been isolated.
Applications in dentistry
Various tissues of dental origin have been considered as well as experimented with for the isolation of stem cells. In the field of dentistry, stem cell research is directed towards achieving the following; - regeneration of damaged coronal dentine, pulp, resorbed roots, cervical or apical dentine and periodontal ligament; besides plugging of perforations, repair of craniofacial defects and whole tooth regeneration.
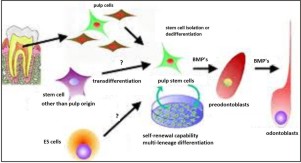
Dental pulp stem cells (DPSCs) and regeneration of damaged coronal dentine and pulp (fig 5) Dental pulp stem cells represent a kind of adult cell colony which has the potent capacity of self –renewal and multiline differentiation (fig 6). The exact origin of DPSCs has not been fully determined. These stem cells seem to be the source of odontoblasts that contribute to the formation of dentin pulp complex. Recently, advancements in stem cell biology and tooth regeneration have enabled us to contemplate the potential applications of DPSCs. Some studies have proved that DPSCs are capable of producing dental tissues in vivo including dentin, pulp and crown like structures, whereas other investigations suggested that these stem cells can bring about formation of bone-like structures. Theoretically, a bio-tooth made from autogenous PSCs should be the best choice for clinical tooth reconstruction[7].
Granthos et al[8] demonstrated, both in vitro and in vivo, in animals that dental pulp stem cells (DPSCs) were capable of forming ectopic dentin and associated pulp tissue. But these techniques need further studies to evaluate their efficacy with clinical trials.
Batouli et al[9]used an in vivo stem cell transplantation system to investigate differential regulation mechanisms of bone marrow stromal stem cells(BMSCs) and DPSCs. DPSCs were found to be able to generate a reparative dentine like tissue on the surface of human dentin in vivo. This study provided direct evidence to suggest that osteogenesis and dentinogenesis mediated by BMSCs and DPSCs, respectively, may be regulated by distinct mechanisms, leading to the different organization of the mineralized and nonmineralized tissues.
Adult dental stem cells can differentiate into many dental components, such as dentin, periodontal ligament, cementum and dental pulp tissue but not into enamel.
Regenerating periodontal ligament
Periodontal regeneration has always remained a challenge as it consists of cells that give rise to both hard and soft tissues. It is evident, that the ligament complex contains stem cells that can commit to a number of pathways (bone, cementum and ligament). Kawaguchi et al (2004) used autologous bone marrow (MSC) in combination with allocollagen to regenerate periodontal ligament in experimental grade III defects in dogs. One month after implantation, there was regeneration of cementum, periodontal ligament, and alveolar bone. This study provided firm evidence that MSC when embedded in the appropriate environmental niche, can be used to regenerate a tissue as complex as the periodontium[10].
Hasegawa et al[11] demonstrated that autologous periodontal ligament cells cultured in vitro were successfully reimplanted into periodontal defects in order to promote periodontal regeneration in dogs and a subsequent study confirmed this evidence in humans.
Bio- Tooth Generation
Replacing a missing tooth has always been a challenge in the field of dentistry. No doubt dental implants have solved the problem to an extent but they rely on direct integration of bone with the implant surface which is indeed an unnatural relationship as compared to that of a natural tooth with bone. Murine stem cells when transferred into renal capsules resulted in development of tooth structure and associated bone. Teeth have also been engineered ectopically and transplanted into the jaw with some success.[12] Recently some researchers developed a bioroot into which a post and crown were placed. This further developed a natural relationship with bone. Complete tooth regeneration can also be accomplished by placing the stem cells into a mold of tooth crown which is made of enamel-like substance with a scaffold material. The stem cells then start looping blood vessels through this scaffold to enable its implantation elsewhere in the body until mature teeth are formed, following which these teeth will be extracted and implanted in the oral cavity[13].
Craniofacial Defects:
Craniofacial bone grafting procedures rely on autologous bone grafting, devitalized allogenic bone grafting and natural/synthetic osseo conductive biomaterials. Stem cell transplantation procedures have been studied to replace these procedures in future[14]. Adult stem cells can be harvested from the bone marrow and expanded in the laboratory. When loaded onto appropriate scaffolds and transplanted back into a deficient site, stem cells have the potential to regenerate bone structures. (Source: Krebsbach PH, Robey PG. Dental and skeletal stem cells: potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002 Jun; 66(6):766-73 (fig 7).
Dental stem cell banking[15],[16]
With the advancement of cryo preservation technology, the first commercial tooth bank was established in 2004 at National Hiroshima University, Japan. Presently, Stem Cell Banks freeze not only cord stem cells but also dental stem cells of baby teeth. This can be done easily when a child’s anterior milk tooth is shedding. The tooth is extracted by the dentist and preserved in a special kit provided from the stem cell bank company which transfers the tooth to their special labs to harvest the dental stem cells and store them in their bank for each child ,confidentially, until they are needed later for the child himself or a member of his family.
Challenges associated with stem cell research
Stem cells obtained from any source are less in number[17]. Isolation, culture and storage are technique sensitive. Their immunomodulatory characteristics are still questionable. As of now autologous stem cells have no risk of immune rejection, are least expensive and have no ethical concerns but the procedure is time consuming. Using allogenic stem cells saves considerable time but the risk of immune rejection and pathogen transmission limits their utility. However there are many in vivo studies which support their immunologically safety[18].
Conclusion
Research involving stem cells in teeth has boomed during the past four to five years. In the near future dental stem cells will probably be able to grow new teeth and jaw bone, thereby enabling unlimitedtherapeutic applications. However various challenges must be overcome before this novel therapy can be translated from labs to clinics. Collaboration between basic scientists and clinicians is required to achieve this goal. Recent advancements in scaffold designing, better understanding of growth factor biology and interactions between allogenic stem cells and immune system have resulted in the new promising discipline of ‘Regenerative dentistry’.
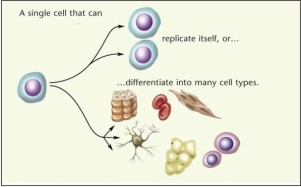 | Fig.1 : stem cell differentiation into many other cell types(Source: Image prepared by catherine Twomey for the national academics, understanding stem cells:An overview of science and issues.)
 |
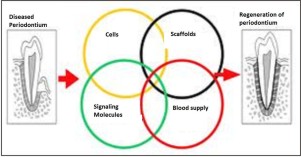 | Fig.2 : Factors involved in tissue engineering and regenerative medicine (Source: Nakashima M, Reddi H. The application of bone morphogenic proteins to dental tissue engineering. Nature Biotech 2003;21:1025–32)
 |
 | Fig.3 : Scaffold in bio-tooth formation. (Source: Columbia University Medical Center)
 |
 | Fig.4 : Tooth development stages and derivation of dental derived stem cells from human primary dental follicle stem cells (DFSC), periodontal dental pulp stem cells (PDLSC), exfoliated deciduous teeth(SHED) ,Dental pulp stem cells (DPSC), stem cells from
 |
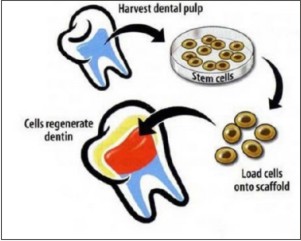 | Fig.5 : Dental pulp stem cells (DPSCs) and regeneration of damaged coronal dentine and pulp(Source: Krebsbach PH, Robey PG. Dental and skeletal stem cells: potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002 Jun; 66(6):766-73)
 |
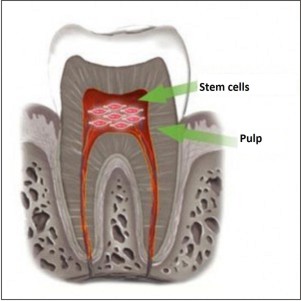 | Fig.6 : Cells will be able to regenerate in future from pulp.(Stem saves: National Institute Of Health: The human body)
 |
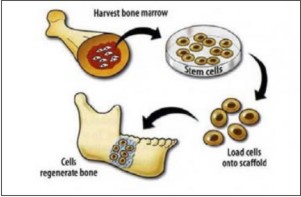 | Fig.7 : stem cells have the potential to regenerate bone structures.(Source: Krebsbach PH, Robey PG. Dental and skeletal stem cells: potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002 Jun; 66(6):766-73).
 |
References
1. Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6; 282(5391):1145-7.
2. Barrilleaux B, Phinney DG, Prockop DJ, O'Connor KC. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006 Nov; 12(11):3007-19.
3. Nakashima M, Mizunuma K, Murakami T, Akamine A. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11). Gene Ther. 2002 Jun; 9(12):814-8.
4. Hutmacher DW, Sittinger M, Risbud MV. Scaffold based tissue engineering: Rationale for compute solid free form fabrication systems. Trends Biotechnol 2004; 22:354-362.
5. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002 Jul 4; 418(6893):41-9. Epub 2002 Jun 20.
6. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003 May 13;100(10):5807-12. Epub 2003 Apr 25.
7. Murray PE, Garcia-Gadoy F, Hargreaves KM. Regenerative endodontics: A Review of current status and a call for action. J Endod 2007; 33:377-390.
8. Granthos S, Brahim J, Fischer W, Cherman N, Boyde A, DenBesten P, et al. Stem cell properties of human dental pulp stem cells. J Dent RES 2002; 81:533.
9. Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al. Comparison of stem cell mediated osteogenesis and dentinogenesis. J Dent Res 2003; 82:976-981.
10. Shi S, Bartold PM, Miura M, Seo BM et.al. The efficacy of mesenchymal tem cells to regenerate and repair dental structures. Orthod Craniofacial Res 2005:8:191-9.
11. Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I. Human periodontal ligament stem cell sheets can regenerate periodontal ligament tissue in athymical rat model. Tissue Eng. 2005; 11:469-477.
12. Ohazama A, Miletech MS, Sharpe PT. Stem cell tissue engineering of Murine teeth. J Dent Res 2004; 83:518-552.
13. Zhang W, Walboomers XF, van Kuppevelt TH, Daamen WF, Bian Z, Jansen JA. The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials. 2006 Nov; 27(33):5658-68. Epub 2006 Aug 17.
14. Abukawa H, Shin M, Williams WB, Vacanti JP, Kaban LB, Troulis MJ. Reconstruction of mandibular autologous tissue-engineered bone. J Oral Maxillofac Surg 2004; 62:601-606.
15. Yen- Hue Huang, Jen-Chang Yang et al. al. Dental stem cells and tooth banking for regenerative medicine.J Exp Cli Med 2010: 2:111-117.
16. Coburn RJ, Henriques BL, Francis LE. The development of an experimental tooth bank using deep freeze and culture techniques. J Oral Ther Pharmacol 1966:2:445-50.
17. Poulsom R, Alison MR, Forbes SJ, Wright NA. Adult stem cell plasticity. J Pathol 2002; 197:441-456.
18. Slifkin M, Doron S, Snydman DR. Viral prophylaxis in organ transplant patients. Drugs 2004; 64:2763-2792.
|