Introduction
Irrigation is one of the most important aspects of root canal preparation. It is presently the best method for the removal of tissue remnants and dentin debris during instrumentation. The simple act of irrigation flushes away loose, necrotic, contaminated materials before they are inadvertently pushed deeper into canal and apical tissues. Irrigation solutions also provide gross debridement, lubrication and destruction of microbes and dissolution of tissues.
During irrigation, hard as well as soft dental tissues are exposed to solutions deposited in the pulp chamber. This may cause alterations on dentin and enamel surface and effect their interaction with materials used for obturation and coronal restoration as well as inhibit resistance to bacterial ingress and permitting coronal leakage[1].
The high concentration of NaOCl is toxic and can cause inflammation in periapical tissues, whereas in low concentrations it is ineffective against specific microorganisms [2]. EDTA solution has a strong demineralizing effect, it causes enlargement of dentinal tubules, softening of dentin and denaturation of collagen fibers and 17% EDTA at pH 7.4 for 10 minutes application caused excessive peritubular and intertubular dentinal erosion [3],[4]. High concentration of hydrogen peroxide caused the great decrease in dentin microhardness & affected the inorganic parts of dentin through acidic demineralization and attacks the organic rich inter-tubular dentin by collagen denaturation action [5].
The objective of this review was to evaluate the effect of various irrigating solutions used in endodontics on the various characteristic properties of dental hard and soft tissue, so that these solutions can be used judiciously and appropriately without harming the pivotal tooth structure. The literature search used the MEDLINE database which goes back to 1957 and was limited to English-language papers. Reference lists of potentially relevant articles and review articles were also screened for the search strategy. The keywords searched were root canal irrigants, endodontics, side-effects, cytotoxicity, smear-layer, dentin hardness, and pulp-dissolution.
Classification of types of chemicals used for root canal irrigation
Effect Of Root Canal Irrigants On Tooth Structure:
Demineralization:
According to Nygaard-Ostby (1957) [6], even lyophobic substances such as dentin, the mineral components of which are mainly phosphate and calcium, are soluble in water. When the disodium salt of EDTA is added to this equilibrium, calcium ions are removed from the solution. This leads to the dissolution of further ions from dentin so that the solubility product remains constant. Thus, chelators cause decalcification of dentin.
It had been found that any change in the calcium ratio may change the original proportion of organic and inorganic components, which in turn changes the microhardness, permeability and solubility characteristics of dentin and may adversely affect the sealing ability and adhesion of dental materials to dentin [7].
The morphological changes in instrumented root canal wall were studied and observed that when final irrigation was conducted with 15%EDTA, the surface dentine appeared smooth but not eroded, and dentinal tubule orifices were regular and separated. However, when 15%EDTA irrigation was followed by 6% NaOCl, peritubular and intertubular dentinal erosion were observed. Dentine in the root canal wall surface was rough in appearance and dentinal tubule orifices were irregularly enlarged due to decalcification of the inorganic component by EDTA and the dissolution of the organic matrix by NaOCl [8]. In agreement with previous studies, it has been inferred the most efficient solublizer of dentin was 17% ethylenediamine tetra-acetic acid, which dissolved nearly 70% of dentin mass. The isotonic saline was least capable, dissolving about 3% of dentin; while 5.25% sodium hypochlorite was able to solublize approximately 22% of dentin [9].
Changes in dentin hardness:
Dentin is complex tissue, with varying degree of calcification in different areas, which in turn affect physical properties. Microhardness measurements in transverse crown and root sections of mature, freshly extracted, human teeth showed that the hardness of dentin near the dentino-enamel junction was about 15 KHN softer than surrounding dentin. The microhardness of dentin adjacent to the pulp chamber was about 30 KHN lower than the surrounding dentin. Dentin surrounding caries lesions had microhardness approximately 10 KHN greater than normal dentin; whereas microhardness values at the center of the lesion were much softer [10].
The irrigation of root canals either with hydrogen peroxide, sodium hypochlorite or EDTA solution decreased the microhardness of root dentin [1]. The dentin hardness is location-related and its value decreased as the indentations were made closer to the pulp; lower KHN values were obtained at level of 500 um compared with 1mm distance from the pulp space. In agreement with previous studies, that the microhardness of root dentin was significantly decreased after irrigation with 2.5% NaOCl solution and reduction of microhardness of inner ring 500 um from the lumen was greater than that of the outer ring i.e. 1000 um from the lumen. Moreover, the efficacy of the irrigation solution also depended on its ability to penetrate and wet intratubular dentin. As the distance the fluid has to penetrate increased, its effect would be reduced. This happened also because the number and size of dentinal tubules decreased in the periphery [11].
Similarly, effect of 30% H2O2 for 24 hours was found to cause the surface changes to intertubular dentin and significantly decreased the hardness of intertubular dentin [12]. A significant change in mechanical properties of dentine may compromise the longevity of teeth due to a decrease in its overall strength and resistance to fracture. In a recent study, the effect of irrigation on the microhardness of root canal dentin with strong acid electrolyte water was evaluated and compared its effect to 5.25% NaOCl, 3% H2O2 & 15% EDTA. The findings were in concurrences with previous results that 5.25% NaOCl, 3% H2O2 & 15% EDTA significantly reduce the microhardness; however, strong acid electrolyte water didn’t alter the microhardness of root canal dentin [13].
Changes in the tensile strength, flexural strength and elastic modulus of root canal dentin:
There is convincing evidence circumstantial evidence for the putative causes of nonvital and root treated teeth to fracture. The main causes are altered physical properties mainly alterations in the tensile strength, microhardness, flexural strength and modulus of elasticity; altered physical properties of dentin and altered proprioception / nociception. In addition to cumulative effect of these factors, endodontic irrigants, medicaments and materials may also play a part in influencing the physical and mechanical properties of dentin. It is probable that these factors interact cumulatively to influence tooth loading and distribution of stresses, ultimately increasing the possibility of catastrophic failures. It can be concluded that 5.25% sodium hypochlorite compared to saline significantly reduced the flexural strength and elastic modulus of dentin [14].
Treatment of dentin bars with 3% and 5% NaOCl solutions caused a significant decrease in their modulus of elasticity and flexural strength while the exposure to Ca(OH)2 significantly reduced the flexural strength but had no significant effect on the modulus of elasticity [15]. Similarly, effect of 30% hydrogen peroxide for 24 hours was found to cause the surface changes to intertubular dentin and significantly decreased the modulus of elasticity of intertubular dentin[12]. A significant change in mechanical properties of dentine may compromise the longevity of teeth due to a decrease in its overall strength and resistance to fracture.
Cleaning efficacy, Smear layer removal and dentin permeability:
There is a controversy as to whether the smear layer should be removed or not before obturation of root canal. Microorganisms can remain in or migrate into dentin despite thorough chemomechanical preparation[16]. Some authors proposed that smear layer acted as a barrier to bacterial metabolites, preventing the bacterial invasion of the dentinal tubules and not as a preferred site for bacterial colonization. However, bacteria not only remained, but also survived and multiplied in the smear layer and can penetrate the dentinal tubules. The antimicrobial action of medicaments in the dentinal tubules could be delayed or hindered by smear layer. This would appear to make smear layer removal advisable [17].
The effect of sodium hypochlorite and hydrogen peroxide was studied and found that the dentin permeability, measured as hydraulic conductance was increased more than twice, when dentin was immersed in 5% NaOCl due abundant dissolution of organic content of the dentin. However, exposure to 35% H2O2 lowered the hydraulic conductance by approximately 16.6% because of formation of amorphous precipitates on the dentin surface [18].
A study conducted revealed that the specimens irrigated with 5.25% NaOCl showed heavy smear layer covering the apertures of dentinal tubules while a thin smear layer covered surface was present in specimens irrigated with 2% chlorhexidine liquid. But, the 2% chlorhexidine gel treated samples resulted in cleanest tubules with almost all the tubules opened[19]. It is also confirmed that in case of 2.5% NaOCl treated samples, the superficial debris was absent on the dentinal walls, but the smear layer was still present and none of the dentinal tubules seemed to be patent. However, 50% citric acid and tetracycline hydrochloride samples revealed that the smear layer was completely removed and dentinal tubules apertures were widened. However, citric acid caused more extensive peritubular dentin demineralization and greater degree of morphological alterations in root dentin than tetracycline [20].
Dissolution of necrotic pulp remnants:
Essential to endodontic success is the careful removal of remnants, microbes and dentinal fillings from the root canal system. The apical portion of root canal is especially important because of its relation to the periradicular tissue. With current instrumenting techniques, an average of 40% to 50% of the root canal wall surface is untouched, leaving ample tissue in which microbiota can survive and regrow. Therefore, irrigation serves as a physical flush to remove debris as well as serving as a bactericidal agent, tissue solvent and lubricant[21]. The solvent effect of irrigating solutions depends on the concentration, pH, volume, temperature, time, exchange / refreshment, mechanical agitation, amount and surface area of tissue & tissue type[22]. Also, 5% NaOCl dissolved the tissue more effectively than 0.5% NaOCl, but pretreatment with Ca(OH)2 solution for 30 minutes enhanced the tissue-dissolving ability of 0.5% NaOCl as it caused the tissue to swell and thus, became more accessible to sodium hypochlorite[23]. In agreement with previous studies, all the sodium hypochlorite solutions dissolved the pulp tissue but chlorhexidine preparations as well as distilled water, were not able to dissolve the fragments. The dissolution speed varied proportionally to the concentration of sodium hypochlorite solution, more concentrated the solution, the higher the speed of dissolution[24].
Hazards of root canal irrigants:
- Cytotoxicity:
Irrigation solutions are used in endodontic therapy to remove debris from the root canal, eliminate microorganisms, and serve as a lubricant during instrumentation. Therefore, an “ideal” irrigation solution should be efficient as an antimicrobial agent but not toxic to the periodontal tissues. During endodontic treatment the irrigating solution will be in contact with pulpal and periapical tissues. Debris as well as irrigating solutions may also be pushed beyond the apical foramen and cause further periapical complications and delayed wound healing[25]. The cytotoxic effect of 10% citric acid and EDTA-T was studied[26] and observed that 10% citric acid, independent of dilution tested, was more biocompatible than EDTA-T because cultures of fibroblasts treated with citric acid had a higher percentage of viable cells.
- Complications during root canal irrigation & its management:
- Inadvertent injection of sodium hypochlorite beyond the apical foramen
- Damage to eyes
- Air emphysema
- Contact dermatitis and various allergic reactions[28]
Conclusion:
It can be deduced from the literature that 2.6% & 5.25% sodium hypochlorite has the unique capacity to dissolve necrotic tissue and the organic components of the smear layer while 17%EDTA, 10% citric acid and MTAD are excellent in removing the inorganic contents of smear layer. Also, all the root canal irrigants significantly alter the physical properties of tooth and thereby, affecting the outcome of treatment. However, further research is required to improve the root canal irrigants and overcome their deleterious effect on tooth structure. It has been found that cytotoxicity of all the irrigants is concentration dependent that is higher the concentration of the root canal irrigants greater will be the toxicity to periodontal ligament and fibroblast ; so in order to overcome this shortcoming it has been suggested to use minimum concentration of the irrigating solutions. It can be inferred from the studies that no irrigant is complete in it and can’t be used as a single irrigant. It has been recommended that in order to gain maximum advantage from the irrigation, the root canal irrigants should be used in combination.
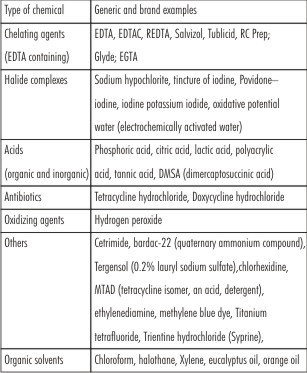 | Table1: various types of root canal irrigants
 |
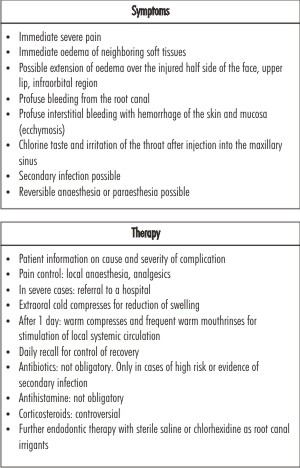 | Table-2: Symptoms and therapy after inadvertent injection of NaOCl into periapical tissues
 |
References:
1. Saleh AA, Ettman WM. Effect of endodontic irrigation solutions on the microhardness of root canal dentin. Journal of Dentistry 1999; 27: 43-26.
2. Leonarda MR, Tanomaru MF, Silva LA, Nelson PF, Bonifacio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. Journal of Endodontics 1999; 25:167-171.
3. Garberoglio R, Becce C. Smear layer removal by root canal irrigants: a comparative scanning electron microscopic study. Oral Surgery Oral Medicine Oral Pathology 1994; 78: 359-367.
4. Meryon S, Tabias R, Jakeman K. Smear layer removal agents: a quantitative study in vivo & in vitro. Journal of Prosthetic Dentistry 1987; 57: 174-179.
5. Lewinstein I, Hirscfeld Z, Rotstein I, Stabholz A. Effect of hydrogen peroxide and sodium perborate on the microhardness of enamel and dentin. Journal of Endodontics 1994; 20: 61-63.
6. Nygaard-Ostby B. Chelation in root canal therapy: ethylenediaminetetraacetic acid for cleansing and widening of root canal. Odontologisk Tidskrift 1957;65: 3-11.
7. Rotstein I, Dankner E, Goldman A, Healing I, Stabholz A, Zalkind M. Histochemical analysis of dental hard tissues following bleaching. Journal of Endodontics 1996; 22: 23-26.
8. Niu W, Yoshioka T, Kobayashi C, Suda H. A scanning electron microscope study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. International Endodontic Journal 2002;35: 934-939.
9. Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, Sodium hypochlorite and EDTA on bovine pulp and dentin Journal of Endodontics 2003; 29: 334-337.
10. Craig RG, Gehring PE, Peyton FA. Relation of structure to the microhardness of human dentin. Journal of Research 1959; 38: 624-630.
11. Goldberg IS, Liberman R, Heling I. The effect of instrumentation with two different file types, each with 2.5% NaOCl irrigation on the microhardness of root dentin. Journal of Endodontics 2002; 28: 311-312.
12. Chng HK, Ramli HN, Yap AUJ, Lim CT. Effect of hydrogen peroxide on intertubular dentin. Journal of Dentistry 2005; 33: 363-369.
13. Qing Y, Akita Y, Kawano S, Kawazu S, Yoshida T, Sekine I. Cleaning efficacy and dentin micro-hardness after root canal irrigation with strong acid electrolytic water. Journal of Endodontics 2006;32:1102-1105.
14. Sim TPC, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentin and tooth surface strain. International Endodontic Journal 2001; 34: 120-132.
15. Grigoratos D, Knowles J, Ng Y-L, Gulabivala K. Effect of exposing dentin to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulus. International Endodontic Journal 2001;34:113-119.
16. Bystrom A, Sundqvist G. Bacteriological evaluation of the effect of 0.5% sodium hypochlorite in endodontic therapy. Oral Surgery Oral Medicine Oral Pathology 1983; 55:307-312.
17. Yamada R, Armas A, Goldman M, Lin P. A scanning electron microscope comparison of high volume final flush with several irrigation solutions. Journal of Endodontics 1984; 9:137-142.
18. Barbosa SV, Safavi KE, Spangberg LSW. Influence of sodium hypochlorite on the permeability and structure of cervical human dentin. International Endodontic Journal 1994; 27:309-312.
19. Ferraz CCR, Gomes BPFA, Zaia AA, Texira BA, Jose de Souza-Filho F. In vitro assessment of antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. Journal of Endodontics2001; 27: 452-453.
20. Lui JN, KuahHG, Chen NN. Effect of EDTA with and without surfactants or ultrasonics on removal of smear layer. Journal of Endodontics 2007; 33: 472-475.
21. Zehnder M, Grawehr M, Hasselgren G, Waltimo T. Tissue dissolving capacity and dentin-disinfecting potential of calcium hydroxide mixed with irrigating solutions. Oral Surgery Oral Medicine Oral Pathology Oral Radiology Endodontology 2003; 96:608-613.
22. Cunningham WT, Balekjian AY. Effect of temperature on collagen dissolving ability of sodium hypochlorite endodontic irrigant. Oral Surgery Oral Medicine Oral Pathology 1980;49: 175-177.
23. Turkun M, Cengiz T. The effect of sodium hypochlorite and calcium hydroxide on tissue dissolution and root canal cleanliness. International Endodontic Journal 1997;30: 335-342.
24. Okino LA, Siqueira EL, Santos M, Bombana AC, Figueriedo JAP. Dissolution of pulp tissue by aqueous solution of chlorhexidine by aqueous solution of chlorhexidine digluconate gel. International Endodontic Journal 2004; 37: 38-41.
25. Chang YC, Huang FM, Tai KW, Chao MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surgery Oral Medicine Oral Pathology Oral Radiology Endodontology 2006; 9: 446-450.
26. Sceleza MFZ, Daniel RLDP, Santos EM, Jaeger MMM. Cytotoxic effects of 10% citric acid and EDTA-T used as root canal irrigants: an in vitro analysis. Journal of Endodontics 2001;27:741-743
27. Krautheim AB, German THM, Bircher AJ. Chlorhexidine anaphylaxis: case report and review of the literature. Contact Dermatitis 2004;50: 113–6.
|