Introduction
Tobacco smoking has a major impact on many tissues and organs of the body, including the periodontal tissues. Cigarette smoking is the single most important and modifiable factor responsible for cases of lung cancer, hypertension, and cardiovascular diseases.[1] Various malignant andpremalignant oral lesionshave been associated with cigarette smoking. Generally speaking, soft tissue conditions, dental caries and delayed wound healing are exceedingly more prevalent in smokers compared with non-smokers.[2]
Periodontal disease has been added to the ever-increasing list of health consequences (oral and systemic) of tobacco smoking.Not only cigarette smoking but also cigar, pipe, and water pipe smoking entail an increased risk for periodontal disease.Chronic exposure to many substances in tobacco and tobacco byproducts significantly affects the prevalence and progression of periodontal diseases.[3] So profound is the negative effect of cigarette smoking on the periodontium that the exposure to second-hand smoke accounts for 30% of periodontal disease in non-smokers. In addition, tobacco use complicates periodontal therapy and substantially reduces the possibility of favorable treatment outcomes. A number of studies of various designs performed in many countries over the last decades unanimously demonstrate that the periodontal health of smokers is greatly inferior to that of non-smokers.
Cigarette smoking accounts for approximately half of periodontitis diagnosed in young adults (<35 years). Smokers are almost three times more likely to show severe periodontal disease compared with non-smokers. Current smokers were also 3.3 times more likely to attend a periodontal practice office compared to non-smokers.[3] The effect of smoking on periodontal tissues is cumulative and dose dependent. Clinically, smoking-associated periodontal disease presents with thick inflamed marginal gingiva and gingival recession. The buccal marginal gingiva of both upper and lower anterior teeth often presents with the characteristics stain of smoker melanosis.[4] The degree of alveolar bone destruction far exceeds the periodontal destruction evident clinically.
Thus, cigarette smoking is the single, modifiable environmental factor responsible for the excess prevalence of periodontal disease in the population. Cases of periodontal disease attributed solely to smoking are by far greater than those owed to other important factors such as diabetes mellitus.
Physiochemical Properties Of Cigarette Smoke
Cigarette smoke is a heterogenous aerosol produced by incomplete combustion of the tobacco leaf. It is composed of a gas phase in which particulate matter is dispersed. In the presence of intense heat of combustion some tobacco constituents undergo thermic decomposition (pyrolysis). Volatile substances are distilled directly into the smoke. Unstable molecules recombine to generate new compounds (pyrosynthesis). Some substances found in tobacco pass unchanged into cigarette smoke.[5] Raw and processed tobacco has been shown to contain more than 2500 different chemical constituents. When smoked, it liberates ammonium compounds, pyridene bases, phenols, tar, nicotine and others (Table 1). The most potent carcinogens in tobacco are the tobacco specific nitrosamines, polycyclic aromatic hydrocarbons, tar etc. NNN (N-nitro-sonornicotine), a volatile N-nitroso compound, has shown tumor-initiating properties in laboratory animals. It is the first organic carcinogen isolated from smokeless tobacco. Increased amounts of the carcinogen have been shown in snuff and chewing tobacco. It is partly derived from bacterial or enzyme action on nicotine.[6]
Among the substances found in tobacco smoke is the alkaloid nicotine, which
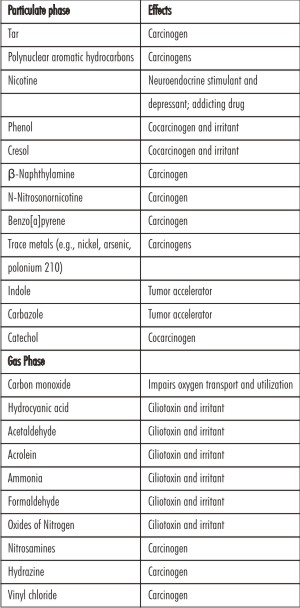 | Table 1: Selected Cigarette Smoke Constituents
 |
appears to be responsible for the dependence that characterizes the smoking habit.[7] During smoking, nicotine is rapidly absorbed into the bloodstream, where 30% remains in its free form. It is highly lipid-soluble and readily penetrates cell membranes.
Nicotine has actions on almost all the organs of the body. Nicotine is considered the most pharmacologically active compound in tobacco smoke. Most is absorbed through the lung alveoli, but nicotine can also be absorbed, though more slowly, through the oral mucosa in sufficient quantities to have a pharmacological effect.[8] Nicotine has pronounced effects on the cardiovascular system as it increases the heart rate, cardiac output, and blood pressure by autonomic stimulation.
Effect Of Smoking On Plaque
Effect of Smoking on Plaque Development
Early observations report that smokers showed a higher prevalence of dental plaque than non-smokers and suggested more severe periodontal disease in smokers might be because of greater accumulations of plaque. However, other studies indicated that, when other factors were controlled, smoking did not appear to increase the amount of plaque.[9]In addition, studies in which the development of plaque and inflammation was observed in an experimental gingivitis model showed that the rate of plaque formation was similar between smokers and non-smokers.[10]
Effect of smoking on the oral and subgingival microflora
Study of microbiota of the oral mucous membranes and saliva failed to establish a statistically significant trend for smokers to harbor greater proportions of putative periodontal pathogens in the oral locations. Anexperimental gingivitis study showed no difference between smokers and non-smokers in the alterations to supra- and subgingival microflora during the change from relative health to experimentally induced gingivitis.[11]
The earliest reported evidence of subgingival microbiological differences between smokers and non-smokers was provided by Zambon et al. (1996). Immunofluorescent microscopy was used to identify the presence of a number of putative periodontal pathogens from two pooled samples per subject. The results indicated a higher prevalence of A. actinomycetemcomitans, T. forsythia and P. gingivalis in the current or former smokers. In particular, the authors reported that the risk of subgingival infection with T. forsythia in current smokers was 2.3 times than that ofnonsmokers. It wasfound that current smokers displayed an increased risk (Odds ratio 4.6) for harbouring T. denticola in periodontal pockets.[12]
Haffejee and Soransky (2001) investigated the prevalence of a large number of species using checkerboard DNA-DNA hybridization in 272 subjects, of whom 50 were current smokers, 98 were past smokers and 124 never-smoked. They reported a higher prevalence of eight species in current smokers than in the other two groups. Higher prevalence of colonization of periodontal pathogens at shallow sites (<4 mm) in current smokers was an important additional observation.[13]
Calculus Formation
Smokers had more calculus than non-smokers, but the effect of smoking was independent of the amount of calculus present. There have been consistent reports of more calculus in smokers than in non-smokers from the earliest epidemiological studies.[14] Some authors reported that significantly more pipe smokers than cigarette smokers had supragingival calculus. This might be because the pH of pipe smoke is higher than that of cigarette smoke, and because pipe smokers circulate the smoke around the mouth, whereas cigarette smoke tends to be inhaled.[15] Moreover, the smoking cycle is much longer in pipe smokers than in cigarette smokers, causing pipe smokers to salivate more.[16] Reports that calculus formation is more abundant in smokers may be due to the increased salivary flow rates.
There is an increased calcium concentration in fresh saliva in smokers following smoking.[17] Nicotine affects the exocrine glands by an initial increase in salivary and bronchial secretions that are followed by inhibition of the secretions. The calcium phosphates found in supragingival calculus are mainly derived from the saliva. The organic components may also arise from this source, the proteins and polypeptides constituting the major fraction.[18] The increased amount of calculus found in smokers might therefore be due to an effect of tobacco smoke upon properties of saliva.
Effect Of Smoking On The Periodontal Tissues
Effect of smoking on gingival blood flow
There is little or no evidence that smoking induces gingival vaso constriction. Early studies of ANUG recognized that many affected individuals were smokers (Pindborg 1947) and it was hypothesized that the necrotic lesion may have caused vasoconstriction induced by nicotine and stress. Baab & Oberg (1978) were first researchers to question the vaso constriction action of nicotine (from cigarette smoking) on gingival tissues. In a Laser Doppler Flow (LDF) study of 12 young regular smokers, they showed that gingival blood flow rose by about 25% during smoking, was maintained for 5 min and then gradually declined to baseline values. This was associated with an increase in heart rate and systolic and diastolic blood pressure. They confirmed that the blood flow to the skin of forearm did decrease slightly, demonstrating the differences in response between peripheral skin response and those in the head and neck. It was interesting to note that 3 subjects felt light headed after smoking, suggesting that the inhalation dose was greater than normally experienced.[19] They showed that the gingival blood flow had significantly increased at 3 days following quitting and further small increases occurred up to 4 and 8 weeks.
Oxygen tension in the gingival tissues
Tobacco smoke contains carbon monoxide, which is detectable in the breath of smokers and can be used to assess compliance in quit-smoking programme. Oxygen saturation of hemoglobin is affected and attempts have been made to measure this in the gingival tissues of smokers and non-smokers. Hanioka et al[20] (2000) showed variable results. In healthy gingiva, smokers did appear to have lower oxygen tension in pockets of 34 non-smokers and 27 heavy smokers with mild to moderate periodontitis. They showed that the pocket oxygen tension was significantly lower in smokers (mean 21.9 mm Hg CI 18.1-25.7) compared with non-smokers (mean 33.4 mm Hg CI 30.5-36.31). This could have an impact on the pocket microflora.
Gingival inflammation and bleeding
Smoking has a long-term chronic effect, impairing the vasculature of periodontal tissues rather than a simple vaso constrictive effect following a smoking episode. The suppressive effect on the vasculature can be observed through less gingival redness, lower bleeding on probing and fewer vessels visible clinically and histologically. This may also have relevance to the healing response with impairment of revascularization.
Some early studies suggested that smokers experienced less gingival bleeding than non-smokers. This observation was confirmed in a comparative study of 10 heavy smokers (at least 20 cigarettes per day) and 10 non-smokers who had similar levels of periodontitis.[21]
The reduced bleeding on probing was further demonstrated in a study by Bergstrom & Bostrom.[22] Gingival bleeding was lower in 130 smokers than 113 non-smokers. They evaluated the number of visible gingival vessels. There was a tendency for fewer gingival vessels at baseline in the smokers and after 14 and 28 days of plaque accumulation, the smokers had approximately half the number of visible vessels compared with non-smokers, despite having similar levels of plaque. The effect of smoking on gingival bleeding has also been shown in subjects on a quit-smoking programme. Niar et al.[23] followed 27 subjects for 4-6 weeks during a verified successful period of quitting smoking and found bleeding doubled (from 16% to 32%) during this period.
Smoking And Periodontal Disease
Periodontitis is defined as "inflammatory disease of supportive tissue of teeth caused by specific microorganisms which lead to progressive destruction of periodontal membrane and alveolar bone, with formation of periodontal pockets and gingival recession. Opinions have been divided about the effect of smoking on chronic inflammatory periodontal disease. Earlier reviews of the epidemiology of periodontal disease concluded that smoking was a possible causative factor. Few studies have conclusively demonstrated any relevant microbiological changes in the periodontal tissues attributable to smoking. They reported an increased risk for smokers to have subgingival infection with P. gingivalis although this was not found to be statistically significant. In this same study the investigators found smokers were 3 times more likely to harbor A. actinomycetemcomitans. [22]
Mahuca and colleagues[24] evaluated the degree of periodontal disease and its relationship to smoking habits in a population of young healthy male Spanish military recruits. They reported higher plaque and bleeding indices in non-smokers although probing depths and attachment loss were greater in smokers. Young smokers diagnosed with aggressive forms of periodontitis were shown to have more affected teeth and a higher mean loss of periodontal attachment than non-smokers with these conditions. Cigarette smokers had significantly greater probing depths and bone loss than non-smokers although no difference was found in relation to tooth mobility.[25]
Bergstrom et al.[26] found smokers not only to have significantly increased probing depths and alveolar bone loss, but also increased tooth mobility. Some studies have also highlighted the dose relationship between the effect of cigarette consumption and periodontal attachment loss. Luzzi et al.[27] previously reported a relationship between alveolar bone loss and tobacco consumption. The findings when they investigated the relationship between cigarette smoking and bone loss in a group of dental hygienists were suggestive of an effect on alveolar bone that was independent of plaque levels. They also reported that this relationship was age-related, suggesting that progression was more significant in younger smokers.
It has been found consistently that smokers suffer more tooth loss than non-smokers. Daniell[28], in study of osteoporosis in 208 women, aged 60-9, found that 75% of non-smokers and 67% of smokers had natural teeth remaining at 50 years of age. Bergstrom and Floderus-Myrhed [29] in their study and Feldman and Calsina[30] in their surveys of periodontal disease, found that cigarette smokers had significantly fewer teeth than non-smokers.
Smoking And Host Response
Nicotine metabolites can concentrate in the periodontium and their effects include the promotion of vaso constriction and the impairment of the functional activity of polymorphs and macrophages. The numbers of neutrophils in peripheral blood are also increased by tobacco use and their migration through capillary walls. The polymorphonuclear leukocyte (PMN) is the most abundant phagocyte found at the site of acute inflammation and probably has an important role in the defence of the marginal periodontal tissues against bacterial invasion. Corberand (1980) found PMN mobility to be severely depressed by a solution of tobacco-smoke concentrate, although phagocytosis and bactericidal activity were not affected. Smokers have higher blood PMN counts than non-smokers and chemotaxis of PMNs from smokers was suppressed relative to nonsmokers.[31] Alani et al.[32] reported lower levels of both salivary elastase and neutrophils in the oral cavity in smokers with periodontitis. Their study demonstrated that oral elastase and neutrophil counts are lower in smokers compared with nonsmokers with similar levels of periodontal disease. Their results also suggest that these values return to non-smoking level after smoking cessation. The passage of fluid through the junctional epithelium into the gingival crevice is markedly increased in gingival inflammation and resembles an inflammatory exudate. It contains leukocytes and plasma
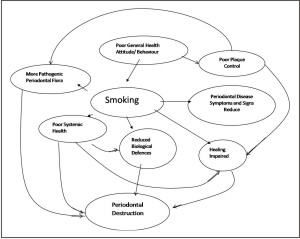 | Figure 1: Diagram Summarizing The Interactions Between Smoking And Other Factors Which Could Ultimately Lead To Periodontal Destruction.
 |
proteins, and probably plays an important role in the defense of the gingival tissues against bacterial attack Smoking appears to reduce the flow of this gingival fluid exudate. Bergstrom and Preber[33] studied 10 smokers and 10 non-smokers over a 4-week period during which the subjects abstained from all oral hygiene measures. They found that the degree of gingival redness, the occurrence of bleeding from gingival margin, and the gingival fluid exudates were less in smokers as compare to non smokers all increased during the experimental period.
A significant genetic component has also been identified in relation to aggressive periodontitis and the combined interaction of cigarette smoking and various genetic polymorphisms might also contribute to disease status in young adults.[34]
Smoking And Periodontal Therapy
Following non-surgical therapy including scaling, root planing and professional tooth cleaning, healing in terms of gingival bleeding reduction and pocket depth reduction was less favorable in smokers as compared to nonsmokers (Figure 1). The clinical results showed a statistically significant reduction of pocket depth and number of diseased sites in both smokers and nonsmokers. These findings are in agreement with recent long-term results which suggest that tobacco smoking interferes with the healing process following non-surgical periodontal therapy.[35],[36] A study by Grossi et al.[37] showed that current smokers have less healing and reduction in subgingival T. forsythia and P. gingivalis after treatment compared to former and non-smokers,suggesting that smoking impairs periodontal healing. Ah MKet al.[38] reported less probing depth reductionand attachment gain in smokers who had been treatedby periodontal surgery, corroborated this finding thatsmokers were poor candidates for successful periodontalcare.A statistically significant difference was observed inthe reduction of probing depth between smokers andnon-smokers at 12 month post-surgical follow-up afterWidman flap surgery on 4 to 6 mm pockets.James and colleagues[39] investigated the in vitroeffect of nicotine on fibroblast activity. They found thatit inhibited attachment and growth of periodontal ligamentfibroblasts. The results of these studies all indicatethat smoking has a deleterious effect on wound healing and may help to explain why smokers respond less favourably to periodontal therapy.
Conclusion
Epidemiological studies of dental disease have consistently found poorer oral hygiene in tobacco smokers than in non-smokers. All of the surveys have reported increased quantities of calculus formation in smokers. It has long been known that smoking causes a marked increase in salivary flow rate as a simple reflex effect and this could explain the tendency of smokers to accumulate increased amounts of calculus. There is some evidence that smoking also increases the mineralizing potential of saliva. All studies that measured plaque in smokers and non-smokers have found more plaque in smokers. There is no evidence that smoking increases the rate at which plaque develops, or that it has any material effect on salivary precipitation. It seems likely that the major factor leading to greater plaque accumulation in smokers is inadequate oral hygiene. Tooth brushing habits in smokers tend to be less favourable than in non-smokers.
Smoking does not ordinarily give rise to striking gingival changes. At a clinical level, smoking appears to suppress visible gingival inflammation in response to plaque accumulation and there is mounting evidence that gingival bleeding is reduced in smokers. Gingival bleeding is an important early sign of chronic gingivitis and the masking of this feature may result in failure to recognize the presence of disease.
Smokers have fewer teeth than non-smokers and more severe destructive periodontal disease; they have deeper periodontal pockets and more alveolar bone loss. Smoking does not ordinarily lead to tooth loss, but the prospect of losing teeth from periodontal disease can be a persuasive argument against smoking.
Tobacco smoke has a strong reducing capacity in the mouth and appears to contribute to anaerobiosis, possibly by altering the oxidation-reduction potential in favour of anaerobic microorganisms, and possibly by a selective toxic effect on particular species. This predisposes smokers to oral infection by anaerobes, such as ANUG, which is associated with proliferation of anaerobic bacteria, is rarely seen in non-smokers, and it could contribute to the progress of destructive periodontal disease.
There is good evidence that smoking depresses the activity of oral PMNs, reducing their chemotactic response, mobility, and phagocytic ability. Blood flow in the gingiva and output of crevicular fluid are also reduced, further decreasing cellular and humoral immune components in the region of gingival crevice. It is not surprising that smoking has recently been shown to impair periodontal wound healing, and this includes nonsurgical as well as surgical therapy. It is hoped that the evidence of the harmful effects of smoking presented in this review might serve to stimulate dentists to give informed advice to help their patients who smoke to stop this deleterious habit.
References
1. Hujoel P.P., Drangsholt M., Spiekerman C., DeRouen T.A. (2002). Periodontitis-systemic diseases associations in the presence of smoking-causal or coincidental? Periodontology 2000. 2002; 30:51-61.
2. National Oral Health Survey and Fluoride Mapping 2002-2003 (INDIA).
3. Bergström J. Tobacco smoking and risk of periodontal disease. J Clin Periodontol 2003; 30:107-113.
4. Francisco Rivera-Hidalgo (2003). Smoking and periodontal disease. Periodontology 2000. 2003; 32: 50-58.
5. Fauci, Branwald, Isselbacher, Wilson, Martin, Kasper, Hauser, Longio. Harrison's Principles of internal medicine (14th Edition) Vol. 1&2.
6. Palmer RM, Mathews JP, Wilson RF. Non-surgical periodontal treatment with and without adjunctive metronidazole in smokers and non-smokers. J Clin Periodontol 1999; 26:158-163.
7. Banoczy J, Gintner Z, Dombi C. Tobacco use and oral leukoplakia. J Dent Educ 2001; 65: 322-326.
8. Wallstrom M, Sand L, Nilsson F, Hirsch JM. The long-term effect of nicotine on the oral mucosa. J Periodontol 1999; 94: 417-423.
9. SheihamA. Periodontal disease and oral cleanliness in smokers. J Periodontol1971; 71:562-567.
10. Bergstrom J. Short term investigation on the influence of cigarette smoking upon plaque accumulation. Scand J Dent Res 1981; 89:235-238.
11. Lie MA, Van der Weijden GA, Timmermann MF, Loos BG, Van Steenbergen TJ and Van der Velden U. Oral microbiotain smokers and non-smokers in natural and experimentally induced gingivitis. J Clin Periodontol 1998,75:557-564.
12. Zambon JJ, Grossi SG, Machtei EE et al. Cigarette smoking infection the risk for subgingival infection with periodontal pathogens. J Periodontol 1996, 67:1050-1054.
13. Haffajee AD & Socransky SS. Relationship of cigarette smoking to the subgingival microflora. J Clin Periodontol 2001; 28:377-388)
14. Martinez-Ganut P, Lorca A, Magan R. Smoking and periodontal disease severity. J Clin Periodontol 1995; 22:743-749.
15. Albandar JM, Streckfus CF, Adesanya MR, Winn DM. Cigar, pipe, and cigarette smoking as risk faktors for periodontal disease and tooth loss. J Periodontol 2000; 71: 1874-1881.
16. Bergstrom J. Tobacco smoking and subgingival dental calculus. J Clin Periodontol 2005; 32: 81-88.
17. Khan GJ, Salah-ud-Din MR, Marwat FM et al. Secretion of calcium in the saliva of long term tobacco users. J Ayub Med Col Abbott 2005; 17: 453-460.
18. Erdemir EO, Erdemir A. The detection of salivary minerals in smokers and non-smokers with chronic periodontitis by the inductively coupled plasma-atomic emission spectrophotometry technique. J Periodontol 2006; 77: 990.
19. Baab DA & Oberg, PA. The effect of cigarette smoking on gingival blood flow in humans. J Clinical Periodontol 1987;14:418-424.
20. Hanioka T, Tanaka M, Takaya K, Matsumori Y, and Shizukuishi S. Pocket oxygen tension in smokers and non-smokers with periodontal disease. J Periodontol 2000;71:550-554.
21. Perber H & Bergstrom J. Occurrence of gingival bleeding in smokers and non-smokers patients. Acta Odontol Scand 1985;43:315-320).
22. Boström L, Bergström J, Dahlén G, Linder LE. Smoking and subgingival microflora in periodontal disease. J Clin Periodontol 2001;28:212-219.
23. Nair P, Sutherland G, Palmer RM, Wilson RF, Scott DA. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol 2003;30:435-437.
24. Mahuca G, Rosales I, Lacalle JR, Mahuca C, Bullon P. Effect of cigarette smoking on periodontal status of healthy young adults. J Periodontol 2000; 71: 73-78.
25. Locker D, Leake JL. Risk indicators and risk markers for periodontal disease experience for older adults living independently in Ontario, Canada. J Dent Res 1993; 72: 9-17.
26. Bergstrom L, Eliasson S, Preber H. Cigarette smoking and periodontal bone loss. J Periodontol1991; 62: 242-246.
27. Luzzi TLI, Greghi SLA, Passanezi E, Santana ACP et al. Evaluation of clinical periodontal conditions in smokers and non-smokers. J Appl Oral Sci 2007; 15: 512-517.
28. Daniell HW. Postmenopausal tooth loss. Arch Inter Med 1983;143:1678-1682.
29. Bergstrom J, Floderus-Myrhed B. Co-twin study of the relationship between smoking and some periodontal disease factors. Comm Dent and Oral Epid 1983; 11: 113-116.
30. Calsina G, Ramon JM, Echeverria JJ. Effects of smoking on periodontal tissues. J Clin Periodontol 2002; 29: 771-776.
31. MacFarlane GD, Herzberg MC, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol 1992; 63: 908-913.
32. Alavi AL, Palmer RM, Odell EW, Coward PY, Wilson RF. Elastase in gingival crevicular fluid from smokers and nonsmokers with chronic inflammatory periodontal disease. Oral Dis 1995; 3: 110-114.
33. Bergstrom J, Preber H. The influence of cigarette smoking on the development of experimental gingivitis. J Perio Res 1986; 21: 668-676.
34. McGuire MK, Nunn ME. Prognosis versus actual outcome. The effectiveness of clinical parameters and IL-1 genotype in accurately predicting prognoses and tooth survival. J Periodontol 1999; 70:394-401.
35. Kaldahl WB, Johnson GK, Patil KD, Kalkwarf KL. Levels of cigarette consumption and responses to periodontal therapy. J Periodontol 1996; 67: 675-681.
36. Heasman L, Stacey F, Preshaw PM, McCracken GI, Hepburn S, Heasman PA. The effect of smoking on periodontal treatment response: a review of clinical evidence. J Clin Periodontol 2006; 33:241-253.
37. Grossi SG, Zambon J, Machtei EE. Effects of smoking and smoking cessation on healing after mechanical periodontal therapy. JADA 1997; 128: 599-607.
38. Ah MK, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL. The effect of smoking on the response to periodontal therapy. J Clin Periodontol 1994; 21: 91-97.
39. James JA, Sayers NA, Drucker DB, Hull PS. Effects of tobacco products on the attachment and growth of periodontal ligament fibroblasts. J Periodontol 1999; 70: 518-525. |