Introduction
Clinical manifestation of periodontal disease depicts past destructive activity which may have caused significant irreparable loss. It would be a nice approach to be able to detect causative periodontal pathogens, intercept and prevent the disease process through practical and rapid tests rather than cure.
The knowledge about the microbiologic and immunologic factors responsible for the pathogenesis of periodontal disease has greatly advanced in the last few years and has improved our understanding of the disease process, leading to the development of certain indicators for the identification of persons and/or sites with higher susceptibility to periodontal breakdown. Recognition and assessment of this susceptibility enhances the predictability and outcome of periodontal therapy.
Evidence has been presented which suggests a specific bacterial etiology in many forms of periodontal diseases. The association of a limited number of bacterial species i.e. Haemophillus Actinobacillus) actinomycetemcomitans (Zamban 1985), P. gingivalis (Slots 1985, Loesche et al 1985) T,(B) forsythia, Wolinella recta (Dznk et al 1985) and spirochetes (Loesche et al 1985) with various forms of periodontal disease allows for the development of diagnostic tools that are based upon the detection of one or more of these in plaque samples[1]. The detection and enumeration of these specific organisms by cultural or microscopic methods is time-consuming and labor intensive. An ideal approach would be the development of a diagnostic test for the presence and level of these organisms that is simple, inexpensive and reliable.
P.gingivalis and capnocytophaga species phenotypically similar to C.gingivalis, T.denticola, a small spirochete (Laughan et al 1982) and T.forsythia Tanner et al 1985) possess a trypsin like enzyme which can be detected by a biochemical chromogenic reaction[2],[3]. Previous studies (Loesche et al 1987) have demonstrated that the hydrolysis of the trypsin substrate N benzyl-Dl-arginase- 2- naphthalamide (BANA) by sub-gingival plaque obtained from a single site correlates best with the numbers and proportions of spirochaetes in plaque samples and may serve as an indicator of clinical disease[4].[21],[25]
Early detection of periodontal disease using BANA hydrolysis provides a rationale for implementing treatment, and allows the operator to intercept the disease at a primary level. The testing of innocuous sites in healthy and diseased individuals would help to clarify their true nature at a cellular level and expose vulnerable areas. This quality would also indirectly enable the clinician to monitor sites for the development of active periodontal disease and even predict future attachment loss. Chair side microbial and enzyme diagnoses offer important advantages by simple, rapid and reliable in-office tests. Based on this data, an attempt is made to detect the BANA positive organisms in periodontally healthy and diseased sites with varying pocket depths[5],[6],[7],[8],[9],[21],[22]
Materials & Methods [10],[11],[12],[13]
1. Mouth Mirror
2. Periodontal probe - Williams graduated periodontal probe
3. Tweezer
4. Sterile Cotton Rolls
5. Sterile curettes - HU FREID Posterior numbers 11/12, 13/14
6. Vortex Mixer
7. Micropipettes from PIPETMAN
8. 0.15mol/L Monopotassium Phosphate
9. 0.15mol/L disodium Phosphate
(The above two solutions are mixed in required proportions to obtain a Sorensen phosphate buffer of pH 7.2)
10. 44mg BANA
11. 1ml dimethyl sulfoxide (DMSO).
12. 99 ml Sorerisen buffer to give u working solution of 0.67 m mol BANA
13. 1 drop of fast garnet indicator dye (0.1 %)
14. Isopropyl Alcohol.
15. Incubator at 37°C[14],[15]
Criteria For Selection [16],[17]:
1. 55 subjects were between the age group of 35-50 years
2. Women who were pregnant or with any hormonal disturbances were not selected.
3. Subjects without any relevant systemic disease were selected.
4. The patient should not have a history of any antibiotic therapy during the past 3 months and should not have undergone periodontal therapy for the past one year.
5. Untreated patients with a clinical diagnosis of chronic periodontitis were selected in addition to subjects with healthy periodontium.
6. All subjects had a full complement of teeth
Patient Categorization:
Group I : (Control Group)
25 periodontally healthy individuals showing sulcus depth less than or equal to 3 mm served as the control group.
Group II (Chronic Periodontitis)
30 patients suffering from chronic periodontitis with a pocket depth of atleast 6 mm were selected, examined and grouped on the day of sampling as they presented themselves at the out-patient section of the department of periodontics. A brief case history was taken and recorded. Each selected site was diagnosed as clinically healthy or diseased depending on the evaluation of the periodontium. All patients were informed of the nature of the study to be undertaken and were willing and co-operative.
Selection of sites:
For the purpose of standardization, the first molar-tooth in each quadrant was chosen. The mesial and distal areas on the buccal aspect in each subject were examined.
Recording of pocket depth :
Probing pocket depth was noted at the selected sites using a William's graduated pocket marking probe. Probing method was standardized by inserting the probe into the mesial and distal areas in the posterior region directed towards the contact point. Weight of the handle was transferred to the tip of the probe to standardize the probing force. However, only the deepest pocket was recorded and the reading made to the nearest millimeter.
Recording of Index:
The following index was recorded for each subject which helped to categorize them into various groups.
Periodontal lndex (PI) by Russel (1956)
This Index was used to estimate the extent of periodontal disease by measuring the presence or absence of the gingival inflammation and its severity, pocket formation and masticatory function.The criteria were used to assess all of the gingival tissue circumscribing a tooth.
A PI score per individual is determined by adding scores of all the teeth and divided by the number of teeth examined.
SCORE CRITERIA
0 Negative - There is neither overt inflammation in the investing tissues nor loss of function due to destruction of supporting tissues
1 Mild gingivitis-There is an overt area of inflammation in the free gingiva, but this area does not circumscribe the tooth.
2 Gingivitis inflammation completely circumscribes the tooth, but there is no apparent break in the epithelial attachment
3 Used when radiographs are available
4 Gingivitis with pocket formation. Tooth is firm with normal function.
5 Advanced destruction with loss of masticatory function. The tooth may be loose, may have drifted, may sound dull on percussion with a metallic instrument, or may be depressed in its socket.
Sampling Procedures:
The first molar tooth in each quadrant was selected for sampling of sub-gingival plaque. The buccal inter-dental areas were chosen and kept as constant. The sample site was isolated with cotton and air-dried. The supra-gingival plaque was removed using a sterile scaler in an apical to coronal direction in order to avoid pushing supragingival plaque into subgingival space.
The area was then instrumented with a sterile curette separately in each of these sites from an apical-most position. The plaque mass obtained on the instrument tip was immediately transferred to a test tube containing 0.6 ml of Sorensen buffer. A minimum of 4 to 6 samples were collected.
Dispersion of the plaque was by vigorous vibration for 20 seconds in a vortex mixer until a homogenous plaque suspension was obtained. 50 microliters of this suspension was then removed with the help of a micropipette and transferred to another test-tube for further analysis.
Enzymatic Procedures:
For the analysis of plaque samples a Sorensen buffer (0.15 mol/I) monopotassium phosphate, 0.15 mol/l disodium Phosphate) at pH 7.2 was used. This solution is required to preserve and maintain the enzymatic activity of the sample.
BANA (Sigma Chemical Company, St. Louis, Missouri, U.S.A.) is a commercially available colourless synthetic peptide in a powder form. It has a strong affinity to proteolytic enzymes like trypsin. It was obtained from Sigma Chemical Company for the purpose of this study.
A stock solution BANA (44 mg) and 1 ml dimethyl sulfoxide (DMSO) was diluted in 100 ml of buffer to give a working solution of 0.67 m mol/l BANA at pH 7 which is said to give the best results for the enzyme assay.100 microlitres (0.1 ml) of this working solution was added to 50 microlitres of the plaque suspension and incubated overnight at 37°C. The period of incubation ranged from 18-20 hours. A drop of 0.1%fast Garnet indicator dye was then added, and the intensity of the chromogenic reaction was read visually and scored as follows[14],[15].
1. Yellow-Negative
2. Yellowish Orange- Weakly Positive
3. Orange red-Positive
4. Red-Strongly Positive
The results for the patients were noted after 5-15 minutes and recorded in a tabular form according to the respective group. They were then subjected to statistical analysis.
Results
The study includes 55 patients, 25 of control group and 30 from chronic periodontitis group. The BANA test results were compared between healthy and diseased sites. Standard statistical techniques like analysis of variance, and chi-square test were used.
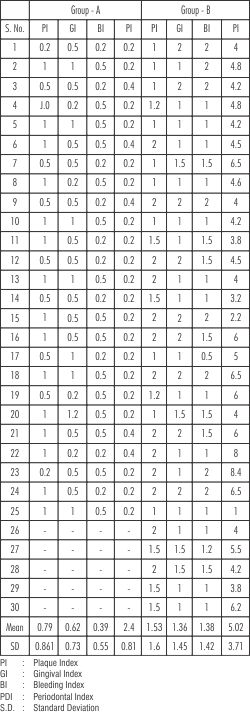
Mean values of plaque, gingival and bleeding index and periodontal index of different groups that were included in this study are presented in Table I.
Plaque Index:
Comparison of mean values of Plaque index are presented in Table 1 & Graph 1. Mean Plaque index in Group A (Control) was 0.79. Group B showed a Mean value of 1.53.
Gingival Index:
The mean values of gingival index in Group I(healthy) was 0.62. It was 1.36 in Group II.
Bleeding Index:
Comparison of mean values of bleeding index in Group I (healthy) was 0.39, Group II revealed l.38.
Comparison between healthy and diseased sites revealed a higher mean value in diseased sites - than in healthy sites.
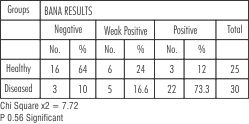
BANA Results:
The BANA results were evaluated at
 | Table 1
 |
 | Table : 2 : Comparison Of Bana Results Between Healthy And Diseased Sites
 |
 | Table : 3 : Specificity And Sensitivity Of Bana Results
 |
control and diseased sites. The results obtained by the BANA test were categorized as :-
Negative - Yellow
Weekly Positive - Yellowish Orange
Positive - Orangish Red
Strongly Positive - Red
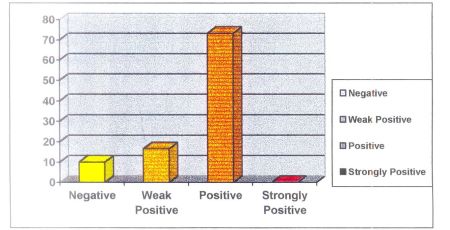
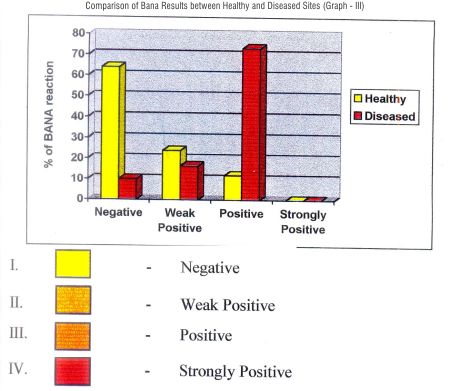
In healthy group 16 sites showed negative, 6 sites showed weakly positive, and 3 as positive.
In diseased group 8 showed negative, 4 sites showed weak positive, 18 sites showed positive results.
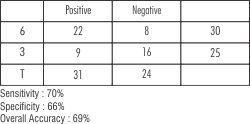
Assessment of validity of BANA test reflected a sensitivity of (true positivity) of 70%, & specificity (true-negativity) of 66% and overall accuracy of 69%.
Discussion
Recent research in periodontology has been devoted to the description of microbial composition of plaque taken from both healthy and diseased subjects and has indicated that a definite number of bacterial species are associated with periodontal disease.This has generated the hypothesis that periodontal disease is essentially an infection due to one or more of the putative periodontopathogens[1],[18],[19],[20],[24]
The bacterial species most frequently associated with periodontitis are anaerobic organisms that can use proteins and peptide as energy sources. Only certain putative periodontopathic bacteria possess a trypsin - like enzyme, that can cleave a variety of synthetic substrates that have arginine attached to a chromophore. When these substrates are incubated with plaque, a color reaction occurs. The hydrolysis of Benzoyl DL Arginase Naphthylamide (BANA) by sub gingival plaque samples was the first of these substrates to be suggested as being of possible diagnostic value in periodontal disease.
The unique nature of BANA hydrolase is the identity of the bacterial species which possess it. Thus far, porphyromonas gingivalis,Tannerella (Bacteroid) forsythia and Treponema denticola are the only bacterial species of over 60 species tested that exhibit strong and consistent BANA hydrolase activity. Apart from these three BANA positive species, Capnocytophaga is also associated with BANA hydrolase activity[2],[3] [21], [22],[23], [25]. However, the capnocytophaga species were identified
 | Graph1
 |
 | Graph2
 |
in low proportions in both the BANA positive and negative results.
The detection of BANA hydrolase positive organisms was done by time consuming procedures like bacterial culture method and immunological assay. For the rapid detection of these organisms in vitro BANA test was first utilized in the dental field by Loesche in 1986.
The following instances are examples of when or where the BANA test could be applied in periodontal therapy.
(a) At initial diagnosis, in conjunction with clinical parameters, so as to establish treatment tactics.
(b) To determine whether initial treatment has been adequate or whether additional modalities are called for.
(c) At recall visits, to determine whether treatment is necessary.
(d) To predict possible future development of periodontal inflammation or periodontal breakdown.
Loesche et al (1987) have shown that BANA hydrolysis by plaque samples has the potential to be a marker of periodontal morbidity as assessed by probing depth measurements and by plaque proportions spirochetes.
Syed et al (1984) have shown that BANA hydrolysis can he used to measure the efficacy of anti-microbial treatment in the experimental gingivitis model.
Gusberti et al (1986) have shown that BANA hydrolysis along with other enzyme markers can be used to diagnose and to monitor treatment efficacy in refractory patients. Thus the clinical management of patients with periodontal disease can be based on criteria given by both bacterial and clinical parameters that can be compared at different time intervals, as was suggested by Listgarten (1986).
The substrate BANA has been used over others as studies have shown that it has a consistent and distinctive ability to reflect the presence of all three pathogens under consideration, did not need the presence of' expensive equipment or expert handling and demonstrated a high degree of sensitivity, specificity, predictivity and accuracy.
The plaque sample is often contaminated with (GCF and or blood, but neither blood, saliva nor GCF are found to be able to hydrolyze BANA.
Limitation of BANA test is, it does not identify which of the three BANA positive species is present in plaque. However, as all three species are anaerobes, it allows the clinician to determine that an anaerobic infection is present.
Research directions regarding the use of the BANA test may include:-
1. Studies to monitor the efficacy of selected clinical or anti-microbial procedures.
2. To assess patient's compliance towards anti-microbial agents.
3. Cost-effectiveness and cost-benefit analysis of the BANA test.
4. Prospective cohort studies, where risk indicators such as a positive test result and the subsequent development of clinical disease could he followed throughout time.
5. School-based screening programmes to identify risk groups for periodontal diseases at an early age.
6. It could also be available for screening of populations or in a manner suitable for epidemiological surveys.
7. It may he applied to assess the microbial status before and after periodontal therapy and also to compare it with other micro-biological diagnostic procedures like bacterial culture, ELISA, and DNA probe.
A tooth site with a BANA positive plaque is indicative of 5 x 10[6] or more of the anaerobic bacteria detected by the BANA test Generally, a weak positive result in a clinically symptom-free patient without a history of periodontal disease confirms the presence of low levels of anaerobic bacteria which are consistent with the absence of periodontal disease for the patient. However, a weak positive result in a symptom-free patient with a history periodontal disease which is now in maintenance phase may be indicative of re-colonization with pathogenic bacteria. Hence, additional preventive measures may be indicated for such a patient. On the other hand a negative BANA response indicates less than 1 x 10[6] of the anaerobic bacteria detected by the test exhibiting clinical judgment of health.[21]
 | Graph3
 |
Similarly the presence of BANA positive plaque around the tooth site at the conclusion of initial periodontal treatment indicates still presence of higher proportions of anaerobic bacteria in the periodontal pocket as a residual infection. This is also indicative of future attachment loss which is having potentially greater clinical significance. Hence, further anti-microbial therapy or surgical intervention or both are essential for the treatment of these.
It has been shown that conversion of BANA positive plaque to BANA negative plaque may lead to a reduction in the need for surgical intervention. And this may be significantly modified by certain host factors. These host factors are host immune responses or patient's ability to maintain the oral hygiene at an optimum level. All these intrinsic and extrinsic host factors determine the tooth's sites specific response to a certain extent.
Thus, the ability of BANA to detect a particular threshold of anaerobic periodontopathic bacteria was found to be a valuable diagnostic tool for screening the individuals at risk for anaerobic infection. BANA test results showing positive or weak positive or negative reaction also has the potential of enabling the clinician to monitor the adequacy of treatment procedures. These findings suggest that BANA hydrolysis by sub-gingival plaque can be used as a simple and objective test to identify those sites in individuals who might require treatment to reduce their pathogenic microflora.
Results obtained from each patient were tabulated and analyzed. A glance at the results the results shows:
Groups-I (Control gp) :
Negative : 16%
Positive : 7-28%
Strongly Positive : 0-8%
Group - II (Diseased)
Negative : 3 : 9.99%
Weakly Positive : 5 : 16.65%
Positive : 14 : 46.62%
Strongly Positive : 8 : 26.64%
The results reported in the study imply that BANA test has multiple utilities in identifying periodontal disease that would facilitate patient management. However, BANA hydrolysis is unable to detect an individual pathogen and their identification via the color reaction or their disappearance after therapy as it is as a group and not individually. Also species associated with active disease like Wolinella recta, E- Corrodens and P-intermedius are not detected. Inspite of these limitations BANA has been shown to be a marker periodontal morbidity as assessed by probing depth measurements and by plaque proportions of spirochetes.
Summary And Conclusion
55 Subjects from a periodontally defined population from the department of periodontics ,Government Dental College and Hospital, Hyderabad were evaluated for the ability of their sub-gingival plaque samples to hydrolyze a 0.67 mmol solution of BANA and correlate it with its clinical diagnosis. Two groups comprising healthy and diseased subjects were tested.
After the clinical assessments were made and the findings recorded, 4 to 6 sub-gingival plaque samples were obtained from the buccal interdental areas around the first molar tooth in each quadrant. After dispersion in 0.6 ml of Sorensen phosphate buffer, 50 microlitres were incubated with 0.1 ml of BANA solution at 370C for 18 hrs. The outcome of the hydrolysis was recorded with the help of colour reaction obtained by the addition of drop of 0.1% solution of fast Garnet indicator dye. It was graded from 1-4 as yellow, yellowish - orange, organic red and red. The purpose of this study was to detect the BANA positive micro-organisms in periodontally healthy and diseased site.
It could be concluded that :
i. BANA is an effective substrate for hydrolysis by specific subgingival plaque micro organisms and can be used as a reliable indicator of BANA positive species in sub-gingival plaque.
ii. The outcome of BANA test was highly significant in periodontally diseased subjects.
iii. The BANA test can he used as a simple and objective means of determining diseased sites requiring periodontal treatment.
iv. It can be used to confirm the need for treatment in patients who have undergone periodontal treatment.
v. It may be used to assess the microbial status before and after periodontal therapy and also to compare it with other microbiological diagnostic procedures such as bacterial culture, ELISA and DNA probe.
Thus, the BANA test has the potential to be used as an objective indicator of future periodontal disease activity in healthy and treated individuals.
References:
1. MOORE W.E., RANNEY R.R. HOLDEMANL V. : Sub-gingival microflora in periodontal disease: Cultural studies. R..J. Genco and S.E. Mergenhagen (eds) , Host-parasite Interactions in Periodontal Disease, P-13, Washington, D.C., American Society of Microbiology, Cited from J. Periodontol 59: 50R-515, 19RR.
2. PEDERSSON E.D, MILLER J. W., MATHESON S., SIMONSON L.G., CHADWICK D.E. COVILL P.J.: Trypsin like activity levels of treponema denticola and porphyromonas gingivalis in adults with periodontitis. J. Clin. Peridontology, 1994 (R): 8;21;519-25.
3. RAMFJORD S.P., CAFFESSE R.G., MORRISON H.C, et al. : 4 modalities of periodontal treatment compared over 5 years. .J. Periodontol 14: 8;445-452, 1987
4. CHENG, S.L. AND CHAN E.C.S.,: The routine isolation, growth and maintenance of the intermediate size, anaerohic oral spirochaete from peridontal pockets. Periodontology. Res. 19: 362-368, 1983
5. LOESCHE W.J. BRETZ W.A., LOPATIN D., STOLL .I., RAU C.F, HILLENBURG K..L KII,LOY W..I., DRISKO C.L, WILLIAMS R., WEBER H.P., CLARK W., MAGNUSSON 1., WALKER C., and HUJOEL P.P. Multicenter clinical evaluation of a chair-side method for detecting certain periodontopathic bacteria in periodontal disease. J. Periodontology, 1990:61; 189-196.
6. LOESCHE W.J.: DNA probe and enzyme analysis in periodontal diagnostics. J. Periodontology 1992:63: 1102
7. LOESCHE W.J., LOPATIN D.E., GIORDANO J., ALEOFORADO G., HUJOEL P. : Comparison of BANA test, DNA probes, and immunological reagents for ability to detect anaerohic periodontal infections due to P.g, T. d. B.f.J., Clin, Microbiol. 1YY2: 30(2) -127-33
8. LOESCHE W..J., SYED S.A. and STOLLS J. : Trypsin like activity in subgingival plaque - A diagnostic marker for spirochaetes and peridontal disease. J. Periodontlogy, 1986: 5 266.
9. SMITH A.J. WADE W.G., GREENMAN J., ADDYM : Analysis of cultivable porphyromonas gingivalis with trypsin like protease enzyme activity and serum antibodies in chronic adult periodontitis. J. Oral Disease. 1995: 1 (2): 70-6
10. BRETZ W.A., W.J. LOESCHE: Characteristics of Trypsin-like activity in sub-gingival plaque samples. Dent. Res. 66: 1668-1672, 1987.
11. Bretz W.A., Lopatin D.E. Loesche W.J., Benzoyl: arginine napthylamide (BANA) hydrolysis by Treponema denticola and or Bacteroid gingivalis in periodontal plaques. Oral Microbiol Immunology. 275-279, 1990.
12. COXS, W. ELEY B.M. Trypase like activity in crevicular fluid from gingivitis and periodontitis: 1. Perio, Res. 1989: 2: 41-44.
13. DRAKE C. W., :: HUNT R.J., BECK J.D., ZAMRON J.J. : : The distribution and interrelationship of Aa, Pg, Pl, and BANA scores among older adults, J. Peridontology, 1993: 64(2): 89-94.
14. FEITOSA A.C. AMALFITANO J, LOESCHE W.J., : The effect of incubation temperature on the specificity of the BANA test J., Oral Microbiol. Immunology 1993: 8 (1): 57-61.
15. JOSEPH AMALFITANO, ANNA B., De FILIPPO BRETZ W.A. and LOESCHE W.J.: The effects of incubation length and temperature on the specificity and sensitivity of the N-Benzoyl- DL-arginine naphthylamide (BANA) J. Periodontology, 1993: 64:848-852.
16. BRETZ W.A., EKLUND S.A., RAD/CCH I R., SCHORK M.A. LOESCHE,. W.J. : The use of a rapid enzymatic assay in the field for the detection of infection associated with adult periodontitis. .J. Public Health Dent. 1993: 53(4): 235:40.
17. FERMIN A CARRANZA., MICHAL G .NEWMAN. : Clinical periodontology, W.B. Saunders Company, 8th Edition, 1996 : 385.
18. LOESCHE W.J., SYED S.A. SCHMIDTHE., MORRISON : Bacterial profiles of sub-gingival plaques in periodontitis. J. Periodontology, 1985: 56-447.
19. CHRISTERSSON, L.A. C.L. FRANSSON, R.G. DUNNFOR, J.J ZAMBON: Sub-gingival distribution of periodontal pathogenic microorganisms in adult peridontitis of. Peridontology 63: -118--125, 1992.
20. GMUR R. STRUB .J.R. GUGGENHEIM B. : Prevalence of bacteroid forsythus and B. Gingivalis in subgingival plaque of periodontally treated patients short recall. J. perio. Res. 1989: 2-1:113-120.
21. The ability of the BANA Test to detect different levels of P. gingivalis, T.denticola and T. forsythia .Braz Oral Res. 2010 Apr-224 Jun;24(2):224-30
22. The ability of the BANA Test to detect different levels of P. gingivalis, T. denticola and T. forsythia. The ability of the BANA Test to detect different levels of P. gingivalis, T. denticola and T. forsythia. J Am Geriatr Soc. 2005 Sep ;53:1532-7 . José Alexandre de Andrade, Magda Feres, Luciene Cristina de Figueiredo, Sérgio Luiz Salvador, Sheila Cavalca Cortelli
23. Periodontal Disease Activity Measured by the Benzoyl-DL-Arginine-Naphthylamide Test Is Associated With Preterm Births. Journal of Periodontology2010, Vol. 81, No. 7, Pages 982-991 , Hui-Chen Chan,* Chen-Tsai Wu,† Kathleen B. Welch,‡ and Walter J. Loesch
24. The prevalence of BANA-hydrolyzing periodontopathic bacteria in smokers. C Kazor, G W Taylor, W J Loesche Clin Periodontol. 1999 Dec ;26 (12):814-21
25. Prevalence of BANA -hydrolying periodontal pathobecteria among smokers & Non smokers with chronic periodontitis.Journal of Dental Science ,Vol- 1;I; 1;june 2010. |