Introduction
Gypsum products probably serve the dental profession more adequately than any other material used in dentistry. Dental plaster, dental stone, high strength dental stone and casting investment material constitute this group of products which are closely related. Dental gypsum products are most widely used among other cast and die materials because of ease of manipulation and other reasonable properties dimensional stability, compatibility with different materials etc.
It is important that cast and die material must have adequate surface hardness to resist abrasion, unfortunately currently available Type I, Type II and Type III do not fill the ideal requirement in relation to hardness which many times results in failure of prosthesis. It has been found possible to produce gypsum products with adequate hardness by incorporation of additives. One method of improving hardness is to impregnate epoxy resin[1] on gypsum although hardening solution may be beneficial[2] but their application involves extra step in cast or die preparation. Studies to reduce the water requirement of dental gypsum products have been conducted to produce set materials with less porosity, greater density and enhanced mechanical properties. Zakaria et al reported some benefit from using two agents," liquid dispersing agent and microcrystal additive,"[3] but the composition of these components was not specified. The addition of a mixture of Gum Arabic and Calcium Hydroxide to Types II and Type III gypsum products has also demonstrated the same effect[4].
It is stated that incorporation of Gum Arabic and Calcium Hydroxide in different proportions like 1% of Gum Arabic and 0.132% of Calcium Hydroxide, 2% of Gum Arabic and 0.2% Calcium Hydroxide will improve the hardness[5]. But there is no specific evidence in the dental literature stating how much percentage of Gum Arabic and Calcium Hydroxide will provide the better hardness to gypsum products.
The present study was planned to compare the surface hardness of Type I, Type II and Type III gypsum products in relation to addition of Gum Arabic and Calcium Hydroxide in different proportions.
Materials and methods
Armamentariums used in this study are-
1. Electronic precision balance
2. Vibrator
3. Volumetric beaker
4. Rubber bowl
5. Straight stainless steel mixing spatula
6. Vicker's hardness testing machine
7. Micrometer microscope
Materials used in this study are-
1. Type I Impression Plaster. [ Ramen research Industry, Kolkata, India]
2. Type II model plaster [Asian Chemicals]
3. Type III dental stone [Asian Chemicals]
4. Gum Arabic [Swastik Pharmaceuticals Mumbai]
5. Calcium Hydroxide [Deepti Dental Products, Ratnagiri, Karnatak]
6. Water
The study was conducted in two phases-
I) Preparation of samples
II) Evaluation of surface hardness.
Standardized rubber moulds ( synthetic rubber-33077 Vulcoform) measuring 1.5 cm height and 1 cm diameter in dimensions were fabricated in a private firm to prepare the uniform size samples for the present study. Selected gypsum products were taken in specified quantity with the help of an electronic precision
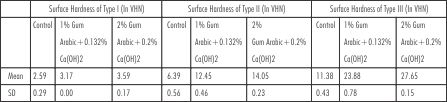 | Table 1 : Comparison of surface hardness of Gypsum Products with different concentrations of Gum Arabic and Calcium Hydroxide
 |
balance. Water was taken in specified volume according to ADA specification No 25 in a volumetric beaker.
I) Preparation of samples-
A. Control Group-
Type I, Type II, and Type III gypsum products and water were taken in a specified quantity of water: powder ratio according to ADA specification No 25 in a clean rubber bowl and manually manipulated to a homogenous mix and vibrated on a vibrator at control speed to remove air bubbles and then poured into the standardized rubber mould. Overall six samples were made. Poured gypsum products were allowed to set for 45 minutes before they were separated from the mould.
B. Preparation of study samples-
Gum Arabic and Calcium Hydroxide (gypsum hardeners were taken in two different proportions for the study purpose-
1. 1% Gum Arabic and 0.132% Calcium Hydroxide ) percent by weight in 100 gms of gypsum powder)
2. 2% Gum Arabic and 0.2% Calcium Hydroxide ) percent by weight in 100 gms of gypsum powder)
The gypsum hardeners were added according to the different proportions in the selected gypsum product after 100% mesh screening. Six samples of each gypsum product were made for each of the two different proportions of gypsum hardeners. Water: powder ratio was taken similar to the control group.
Overall, 18 samples of the control and 36 samples of the study were made and numbered accordingly.
II) Evaluation of surface hardness-
The surface hardness was evaluated after 24 hours of pouring the gypsum products by an experienced engineer who was blinded to the samples. The surface hardness of samples were tested by using Vicker's hardness testing machine (Avery Denison Model 6408, England). This tester consists of a 136 degree diamond pyramid indenter, which contacts and penetrates the surface of a sample under a definite load application. The indenter produces a pyramidal indentation, the diagnoses of which were measured with a micrometer microscope. The weight applied was 10 Kg for 10 seconds and weight applied and time applied were kept constant for all the samples.
The values obtained were compared and subjected to statistical analysis.
Results
Mean and standard deviation of surface hardness of gypsum products Type I, II and III with the addition of Gum Arabic and Calcium Hydroxide in two different proportions are presented in [Table 1]. In all three types of gypsum products the addition of 2% Gum Arabic and 0.2% Calcium Hydroxide showed the highest hardness value followed by 1% Gum Arabic and 0.132% Calcium Hydroxide and the least value was shown by the control group.
One way ANOVA and Student-Newman-Keul's test was used to compare the hardness of Type I, II and III gypsum products in three experimental groups (Table 2). There was a statistically significant difference between type I, II and III in all the three groups as expected. The critical value of F being 3.68 (for all groups) for p=0.05.
One way ANOVA and Student-Newman-Keul's test was used to compare the hardness produced by different concentration of Gum Arabic and Calcium Hydroxide in each type of gypsum. These showed a statistically significant difference in hardness caused by the addition of different concentrations of Gum Arabic and Calcium Hydroxide within each type. The addition of 2% Gum Arabic and 0.2% Calcium Hydroxide showed the highest value among all the three types (Table 3). The critical value of F being 3.68 (for all groups) for p=0.05.
Since all the three gypsum products as well as two proportions of additives showed significant difference in surface hardness, interaction between the products and proportions were also checked by 2-way ANOVA analysis. It revealed that there was a statistically
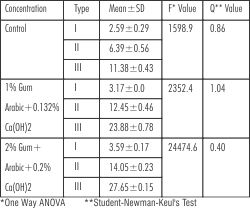 | Table - 2 : Comparison Of Surface Hardness Between Three Types Of Gypsum Products Within The Three Experimental Groups
 |
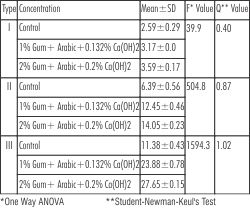 | Table-3 : Comparison of Surface hardness between the experimental groups in Type I, Type II and Type III Gypsum products
 |
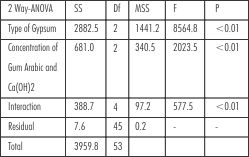 | Table 4 : Results of 2-way ANOVA testing effect of type of gypsum product, concentration of additive and their interaction on surface hardness.
 |
significant difference in hardness resulting from interaction between type if gypsum and the proportion of Calcium Hydroxide and Gum Arabic added. This was contributing to the difference in hardness caused by type of gypsum product and the concentration of additives.
Type III gypsum products with the proportion of 2% Gum Arabic and 0.2% Calcium Hydroxide showed the highest value and least value was shown by Type I control group.
Discussion
Surface hardness is the result of interaction of numerous properties. Among the properties that influence the hardness of the material are its strength, proportion limit, malleability and resistance to abrasion. Numerous factors influence the hardness, so the term is difficult to define.
According to Skinner, hardness is "resistance to indentation"[6]. Surface hardness of gypsum indicates to what extent the forces applied during work on the gypsum cast can be resisted. But studies have shown that surface hardness of these materials is less and not enough to resist the abrasion, so loss of surface details during fabrication leads to error in prosthesis. To overcome this, several methods have been proposed to increase the surface hardness satisfactorily.
Various studies are done by using chemical substitute like epoxy resin1, commercially available model sealants[7], cyanoacrylate[8] and lignosulphonates[9] etc. to increase the surface hardness of gypsum products. Toreskorg et al[2] proved that liquid hardeners increase the surface hardness, Hollenbaek and Sullivan[10] found no such increase with the liquid hardener. However, they reported a dimensional increase in connection with the employment of gypsum hardeners.
Masson[11] described a technique for impregnating stone die with acrylic resin; but Eshleman[12] reported that with acrylic resin there is an increased average die size of 11.7 mm[2].
Alsadi Sally[5] stated the use of gypsum hardening solution that are applied to the set material may be beneficial but their application takes an extra step in cast or die preparation. So, he opted another method of improving surface hardness by addition of Gum Arabic and Calcium Hydroxide in gypsum products.
Gum Arabic is a carbohydrate, Gum hydrolyzing to arabinose and hexoses, found naturally in union with Calcium, potassium, magnesium ions. Calcium oxide when comes in contact with water or moisture, it gives rise to Calcium Hydroxide.
Mixture of Gum Arabic and Calcium oxide markedly reduces the water requirement when used correctly and it is consequently possible to use then in a process for producing ultra hard cast system[13],[14], when small amount of surface active materials like Gum Arabic and Calcium Hydroxide are added to hemihydrates, water requirement of plaster and dental stone are reduced while mechanical properties are improved[6],[15].
Calcium sulfate hemihydrates is ionic in nature, it would be expected that polar-non polar substances would be absorbed by the polar end, the less polar parts being left exposed to the liquid. Surface active materials have a number of hydrophilic groups, which are active in reducing the water requirement. Large molecules with many polar groups may increase the consistency by being simultaneously adsorbed on the two particles of hemihydrates and hence increasing adhesion[13], [14].
Conclusion
From the results obtained by the present study, it has been proved that there is significant increase in the surface hardness by the addition of Gum Arabic and Calcium Hydroxide. All these materials including gypsum hardeners are cheap and easy to manipulate. There is no extra step for die or cast preparation as with other materials like a polysterene, epoxy resins etc. As gypsum hardeners were added directly to gypsum products. All the used materials including gypsum hardeners are cheap and easy to manipulate. The used gypsum hardeners decreases the water requirement, so that the reduction of water-calcined gypsum ratio provides the most practicable means of producing harder casts, the enhanced hardness being due to increased density. Even it has been noted that the surface hardness was increased significantly for dental plaster and dental stone but there was minimal change in hardness of impression plaster. Type III gypsum product with the proportion of 2% Gum Arabic +0.2% Calcium Hydroxide showed the highest value and least value was shown by Type I control group.
To conclude, Gum Arabic and Calcium Hydroxide decrease the water requirement, so that the reduction of water-calcined gypsum ratio provides the most practicable means of producing harder cast. Moreover, further studies are required to know the effect of hardeners on the various physical properties of the gypsum products and also to know the correct water proportions.
References
1. Sanad ME, Combe EC, Grant AA: Hardening of model and die materials by an epoxy resin. J Dent 1980 Jun; 8(2):158-62.
2. Toreskog S, Phillips RW, Schnel R: Properties of die material: A comparative study. J Prosthet Dent 1966; 16:119-31.
3. Zakaia MR, Johnston WM, Reisbick MH, Campagni WV. The effects of a liquid dispersing agent and a microcrystalline additive on the physical properties of type IV gypsum. J Prosthet Dent 1988 Nov; 60(5):630-7.
4. Sanad ME, Combe EC, Grant AA: The use of additives to improve the mechanical properties of gypsum products. J Dent Res 1981; 61:808-810.
5. Alsadi S, Combe EC, Cheng YS.: Properties of gypsum with the addition of gum Arabic and calcium hydroxide. J Prosthet Dent 1996 Nov; 76(5):530-4.
6. Anusavice JK, Brantley AW. Physical properties of Dental Materials. In, Anusavice KJ (ed). The science of Dental materials, 11th edition. London, Saunders, 2004; 96.
7. Sanad ME, Combe EC, Grant AA: The effect of model sealant solution on the properties of gypsum. J Dent 1980 Jun; 8(2):152-7
8. Fukui H, Lacy AM, Jendresen MD: Effectiveness of hardening films on die stone. J Prosthet Dent 1980:44:57-63.
9. Combe EC, Smith DC: Improved stone for construction of models and dies. J Dent Res 1971 Jul-Aug; 50(4):897-901.
10. Hollenbaek GM, Sullivan M. Water substitutes for mixing gypsums. J South Calif Dent Assoc 1964; 32, 199-203.
11. Mason HJ. Impregnation of stone dies with acrylic resin. J Prosthet Dent.1970 Jan; 23(1):96-8.
12. Eshleman JR. Surface hardness and dimensional accuracy of stone dies impregnated with acrylic resin. J Dent Res 1971 Mar-Apr; 50(2):507.
13. Ridge MJ, Boell GR: the water requirement of calcined gypsum. Commonwealth scientific and Industrial research organization. 1962: report F1-7:1-11.
14. Ridge MJ, Boell GR: the water requirement of calcined gypsum. Commonwealth scientific and Industrial research organization. 1962: report F1-9:1-21.
15. Craig RG. Gypsum Products and Investments. In, Craig RG (ed). Restorative dental material, 9th edition. London, Mosby, 1993; 349. |