Introduction
The past decade has seen a veritable explosion of research into the clinical applications of lasers in dental practice, and the parallel emergence of organizations to support laser dentistry with an international focus. The contemporary lasers, however, offer an opportunity to deliver hard and soft tissue treatment that, at least in outline, makes the patient experience somewhat easier than conventional methods. Although lasers can be used in many clinical situations in orthodontics, there are a limited number of literature references mentioning their use, efficiency and cost to benefit ratio versus conventional methods. Thus, this article is an attempt to update the knowledge on lasers, their harmful effects and various possible uses in clinical orthodontics.
History:
The theoretic principles behind the laser were developed as early as 1917, when Einstein laid the groundwork for stimulated emission in his treatise “On the Quantum Theory of Radiation.” [1] The ruby laser became the first medical laser when it was used in 1963 to coagulate retinal lesions. The first gas laser, which was also developed in 1961, used a mixture of helium and neon. Although its beam was not powerful enough to trigger a thermal reaction, its red color allowed it to be used as an aiming beam for invisible lasers such as the carbon dioxide (CO2) laser. Currently, it is marketed in Europe and Asia as a biostimulator to relieve pain and accelerate wound healing.
In 1961, a laser generated from crystals of yttrium-aluminum-garnet treated with 1-3% neodymium (Nd:YAG) was developed. Today, the Nd:YAG laser is mainly used to ablate tattoos and tumors of the genitourinary and gastrointestinal tracts, although it has many other uses, including ophthalmic surgery (e.g., peripheral iridectomies/iridotomies, postcataract capsulotomies) and hair removal.
In 1962, the argon laser was developed. The argon laser is used to photocoagulate (i.e., thermally obliterate without vaporization) blood vessels in the treatment of diabetic retinopathy and port-wine stains. In 1964, Patel at Bell Laboratories developed the CO2 laser. The energy generated by this laser can be used for cutting or volume ablation by means of tissue vaporization. This unique characteristic makes the CO2 laser the most widely used medical laser today.
Laser delivery systems
The coherent, collimated beam of laser light must be able to be delivered to the target tissue in a manner that is ergonomic and precise. There are two delivery systems that are commonly used. One is a flexible hollow waveguide or tube that has an inferior mirror finish. In this system, the laser energy is reflected along this tube and exits through a handpiece at the surgical end with the beam striking the tissue in a non-contact fashion.
The second delivery system is a glass fiber optic cable that is lighter in weight, more pliant and has a smaller diameter than the waveguide. This fiber system can be used in non-contact or contact mode. Most of the time it is used in contact fashion, directly touching the surgical site.
Classifications of lasers
A. According to their mode of emission
a) Fractioned
b) Continuous
c) Pulsed
B. According to their power
a) High power laser
b) Medium power laser
c) Low power laser
C. According to the emitting material
a) Gas laser
b) Solid state laser
c) Dye laser
d) Semiconductor diode laser
e) Ring laser
D. According to the type of tissue being acted upon
a) Hard tissue lasers - The hard tissue lasers are used to cut precisely into bone and teeth, to prepare teeth surfaces for bonding, to remove small amounts of tooth structure, and to repair certain worn down dental restorations.
b) Soft tissue lasers - Soft tissue lasers penetrate soft tissue while sealing blood vessels and nerve endings. This is the primary reason why many people experience virtually no postoperative pain following the use of a laser. Also, soft tissue lasers allow tissues to heal faster.
E. According to their potential of causing biological hazard
a) Class I- These lasers are found in compact disc (CD) players and laser caries detectors. Viewing these lasers with naked eye does not implicit any risk. The maximum power output of these lasers is 40μW for blue light emission and 400μW for red light emissions.[2]
b) Class II- These lasers are found in laser pointers. There are risks in viewing light emissions, both to the naked eyes and when using magnification.[3],[4] The maximum output of these lasers is 1 mW.
c) Class III- These are three subtypes like Class IIIa, IIIb and IIIr. Class IIIa lasers can emit any wavelength and when viewed momentarily, they are non hazardous to an unprotected eye. Class IIIb lasers are lasers with a maximum power output of 0.5 mW. Examples include 'soft' medical lasers, laser light show equipment and laser measuring devices. Environmental controls, protective eyewear, appointment of assigned safety personnel and training in laser safety are required by personnel using these lasers.[5] These lasers can be hazardous to unprotected eyes if viewed directly or from reflective light for any duration. Class IIIr are lasers with lower power outputs than IIIa, and include like low-level medical devices and targeting lasers. For emission in the visual range of wavelengths (400-700 nm), the maximum power output is 5 mW and 2 mW with invisible radiations. The same safety measures are required as with class IIIb lasers.[6]
d) Class IV- This class includes all high-powered, surgical and other cutting lasers. There is no upper limit of power output. All surgical lasers used in dentistry and in oral and maxillofacial surgery are included in this group. The protective measures applicable to class III lasers are further endorsed with the additional risk of fire hazards, due to flash-point temperatures being reached in chemicals used adjunctively to surgical procedures. This group of lasers represents the greatest risk of damage, both to unprotected persons and target tissues, either through direct or reflected and scattered beams.[7]
Basic types of lasers:
Three types of lasers are available for use in dentistry: the CO2 laser, the erbium laser, and the diode laser. The CO2 laser does not contact the tissue during the cutting phase; thus there is no tactile feedback during the surgical incision. It operates with a wavelength that is invisible to the eye, so the fiber optic delivery system has a helium-neon (He-Ne) laser with a wavelength of 632 nm incorporated as an aiming beam. There is slight delay between when the incision is made and when it can be seen. The erbium laser has a wavelength of 2790 to 2940 nm, which makes it ideal for absorption by both hydroxyapatite and water. It can also be used to cut soft tissue, but it does not control bleeding. The diode laser has a wavelength of 812 to 980 nm, which is in the same range of the absorption coefficient of melanin. The laser energy is absorbed by pigmentation in the soft tissues, and this makes the diode laser an excellent hemostatic agent. Because it is used in contact mode, it also provides tactile feedback during the surgical procedure. The diode laser can often be used without anesthesia to perform very precise anterior soft tissue esthetic surgery or surgery in other areas of the mouth without bleeding or discomfor.[8]
Applications of lasers in orthodontics:
Esthetic contouring of the gingival scaffold within the smile framework
Important considerations in finishing our orthodontic patients in terms of smile esthetics now include concepts that are important in cosmetic dentistry-crown heights, tooth proportionality, and gingival shape and contours-therefore, the uses of soft tissue lasers in orthodontic practice broadly fall into the following categories: (1) improving
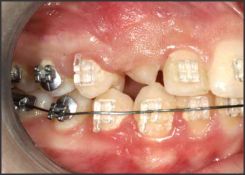 | Fig 1. Partially Erupted Tooth
 |
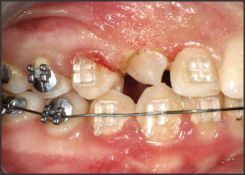 | Fig 2. Removel Of Tissue For Bracket Placement
 |
gingival shape and contour, (2) lengthening crowns, (3) idealizing tooth proportionality, and (4) resolving crown/height asymmetries[9].
Establishing tooth proportionality before bracket Placement
In smile design, our concepts of set formulas for bracket placement depend on the final incisor placement in the dynamic smile, determined by both the incisal edge and the gingival margin. It is therefore important to be able to visualize the crown in ideal proportion before bracket placement. The disproportion could be due to lack of incisor height (gingival encroachment or delayed or incomplete passive eruption requiring gingival reshaping) or incisors that are morphologically wider than ideal. To improve tooth proportion laser gingivectomy could be performed before bracket placement so that we could maximize our chances of positioning the incisors in their ideal vertical position. Because the soft tissue laser seals the incision as it is made, brackets can be placed immediately after the procedure, and healing of the tissue follows.
Crown lengthening
Excellent application of crown lengthening is when a canine is substituted for a congenitally missing lateral incisor. When the first premolar is in the canine position, its crown height looks too short. An option is to lengthen the premolar crown by laser gingivectomy or simplified osseous crown lengthening.
Crown height asymmetry
If the smile line is asymmetric because of differential crown heights between the maxillary right central and lateral incisors, relative to the left side. Using the laser, excision of the gingiva on the right central and lateral incisors could be done, to make the smile more symmetric and greatly improved.
Contouring of gingival and interdental margins
Hypertrophic gingival margins are often seen in orthodontic treatment secondary to marginal gingival inflammation, both acute and chronic. The hypertrophic papillae could be ablated and the rolled gingival margins beveled to sharpen their contour adjacent to the crown of the tooth.
Four weeks after the procedure, the gingival margin greatly improved.
Soft tissue lasers are also used during minor surgical procedures like exposure of impacted teeth, frenectomy, operculectomy and atraumatic Implant placement procedures in orthodontics as (1) the laser cut is more precise than that of a scalpel, (2) the cut is more visible initially because the laser seals off blood vessels
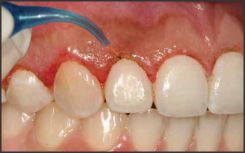 | Fig 1. Contouring Of Gingival Tissue
 |
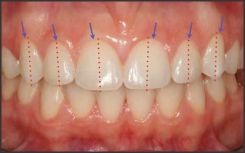 | Fig 2. Gingival Form
 |
and lymphatics, leaving a clear dry field, (3) the laser sterilizes as it cuts, reducing the risk of blood-borne transmission of disease, (4) minimal postoperative pain and swelling have been reported, (5) less postoperative infection has been reported because the wound is sealed with a biological dressing, (6) less wound contraction occurs during mucosal healing, thus scars do not develop, and (7) less damage occurs to adjacent tissues.[10]
Laser etching of enamel for orthodontic bonding
Various commercially available laser systems have been introduced for dental use. Enamel and dentin surfaces etched with erbium, chromium-doped: yttrium scandium- gallium-garnet (Er,Cr:YSGG) lasers show microirregularities and no smear layer.[11] Laser etching inhibits caries, and this could be of great importance in orthodontics.[12] Because water spraying and air drying are not needed with laser etching, procedural errors can be reduced and time saved.[13] Therefore, Er,Cr:YSGG laser irradiation could be a suitable technique to etch enamel for orthodontic bonding.
Pain reduction in Orthodontics with the use of low level laser therapy
Pain from orthodontic treatment is mostly local and therefore may be controlledmore efficiently by locally administered analgesic treatment. One suggested method to control pain is laser therapy.[14], [15], [16], [17], [18]. Two types of laser have recently become available for dental applications. One type, which includes CO2 and Er:YAG lasers, is absorbed by only a thin surface layer of tissue. The other type penetrates into deeper tissue and includes Nd:YAG, He:Ne, and semiconductor lasers.
Several studies have reported analgesic effects of the tissue-penetrating Nd:YAG, He:Ne, and semiconductor lasers for reducing orthodontic pain. Local CO2 laser irradiation will reduce pain associated with orthodontic force application without interfering with tooth movement.
Three - dimensional laser scanning system
Our understanding of the growth of craniofacial features is improving with the development of accurate, low-cost, 3-dimensional (3D) imaging systems, which can be classified as destructive or nondestructive devices,[19] hard or soft tissue imaging devices,[20] and contact or noncontact devices.[21] The laser scanner can be used as a soft tissue scanner and is a valuable tool for its ease of application and creation of 3D images. Images have been created to establish databases for normative populations[22] and cross sectional growth changes,[23] and also to assess clinical outcomes in surgical [24], [25], [26], [27], [28], [29], [30] and nonsurgical treatments [31], [32], [33] in the head and neck regions.
Laser Fluorescence
Enamel demineralization with white spot formation on buccal surfaces of teeth is a relatively common side effect from orthodontic treatment with fixed appliances.[33], [34], [35] Treatment with fixed appliances makes conventional oral hygiene for plaque removal more difficult, thus increasing the cariogenic challenge on surfaces that normally show a low prevalence of dental caries.[36] White spot formation has been attributed to the effect of prolonged accumulation and retention of the bacterial plaque on enamel surfaces adjacent to the appliances. There is evidence, however, thatsuggests that such small areas of superficial enamel demineralization may remineralize.[37], [38], [39]
Sensitive methods that enable early detection and quantification of caries lesions make it possible to monitor changes in the enamel over time. Laser light induced fluorescence as a diagnostic method for detection of enamel caries at an early stage was introduced in 1982.[40]
When enamel demineralization takes place, minerals will be replaced mainly by water causing a decrease in the light path in the tooth substance. This will result in reduction of light absorption by enamel.[41] Because fluorescence is a result of absorption, the intensity of fluorescence will decrease in demineralized regions of the enamel, which appear darker than the sound tooth structure.[42]. Studies have indicated that changes in mineral contents of enamel lesions may be accurately recorded with the laser fluorescence method.
Apthous ulcer management
For patients with apthous ulcer, the diode laser can be used in noncontact mode (1 to 2 mm away from the tissue) to irradiate the ulcer for approximately 30 seconds. The patient reports an immediate reduction of pain. The procedure creates a nonpainful laser wound that replaces the painful ulceration.
Recent researches of lasers used in craniofacial region
Apart from the above uses, the diode laser has also been tried in experimental animals for controlling the excessive growth of the mandibular condyle. It was found that a laser is effective in regulating facial growth and could be a substitute for current conventional methods such as a chin-cup.[39]
It was proposed earlier that orthodontic treatment might cause a decrease in blood flow to the pulp. [44] McDonald and Pitt Ford found that human pulpal blood flow was decreased when continuous light tipping forces were applied to a maxillary canine. [45] Understanding the effects of orthodontic force on the pulp is of particular importance, especially because altered pulpal respiration rate,[46] disruption of the odontoblastic layer, [47] pulpal obliteration by secondary dentin formation, root resorption, [48] and pulpal necrosis [49] have all been associated with orthodontic treatment. Nowadays, laser-doppler flowmetry is a commonly used method to determine the pulpal blood flow. Barwick and Ramsay evaluated the effect of a 4-minute application of intrusive orthodontic force on human pulpal blood flow with laser-doppler flowmetry and concluded that pulpal blood flow was not altered during the application of a brief intrusive orthodontic force. [50]
Recent studies have demonstrated that low-energy laser irradiation stimulates bone formation in vitro and in vivo. Macrophage colony-stimulating factor (M-CSF) is essential and sufficient for osteoclastogenesis. Low-energy laser irradiation stimulates the velocity of tooth movement via the expressions of M-CSF. [47]
Dental laser safety
Safety is an integral part of providing dental treatment with a laser instrument. There are three facets to laser safety, namely:
a) The manufacturing process of the instrument
b) Proper operation of the device
c) Personal protection of the surgical team and patient
Summary
The use of lasers in orthodontics, and in particular diode lasers,has made it possible for orthodontic clinicians to more easily and ably address the challenges faced on a daily basis in orthodontic practice. With nearly a decade of experience using lasers, When used properly, lasers are effective and safe for softtissue procedures and contribute to aesthetic outcomes for orthodontic patients.
References:
1. Einstein A. Zur Quantentheorie der Strahlung. Physiol Z. 1917;18:121-8.
2. Parker S. Laser regulation and safety in general dental practice. Br Dent J 2007; 202: 527-531.
3. Sethi CS, Grey RH, Hart CD. Laser pointers revisited: a survey of 14 patients attending casualty at the Bristol Eye Hospital. Br J Opthalmol 1999; 83: 1164-1167.
4. Robertson DM, McLaren JW, Salomao DR, Link TP. Retinopathy from a green laser pointer: a clinicopathologic study. Arch Opthalmol 2005; 123: 629-633.
5. Reidenbach HD, Dollinger K, Hofmann J. Field trials with low power lasers concerning the blink reflex. Biomed Tech (Berl) 2002; 47 (Suppl 1): 600-601.
6. Chandra P, Azad RV. Laser rangefinder induced retinal injuries. Indian J Opthalmol 2004; 52: 349-351.
7. Schuele G, Rumohr M, Huettmann G, Brinkmann R. RPE damage thresholds and mechanisms for laser exposure in the microsecond-to-millisecond time regimen. Invest Opthalmol Vis Sci 2005; 46: 714-719.
8. David M. Sarver and Mark Yanosky. Principles of cosmetic dentistry in orthodontics: Part 2. Soft tissue laser technology and cosmetic gingival contouring. Am J Orthod Dentofacial Orthop 2005;127:85-90 Ishikawa I, Aoki A, Takasaki
9. Sarver DM. Principles of cosmetic dentistry in orthodontics: part1. Shape and proportionalityof anterior teeth. Am J Orthod Dentofacial Orthop 2004;126:749-53.
10. Soft-tissue lasers in orthodontics: An overview. Neal D. Kravitz and Budi Kusnoto. Am J Orthod Dentofacial Orthop 2008;133:S110-4.
11. Hossain M, Nakamura Y, Yamada Y, Kimura Y, Matsumoto N, Matsumoto K. Effects of Er,Cr:YSGG laser irradiation in human enamel and dentin: ablation and morphological studies. J Clin Laser Med Surg 1999;17:155-9.
12. Klein AL, Rodrigues LK, Eduardo CP, Nobre dos Santos M, Cury JA. Caries inhibition around composite restorations by pulsed carbon dioxide laser application. Eur J Oral Sci 2005;113: 239-44.
13. U¨ sümez S, Orhan M, U¨ sümez A. Laser etching of enamel for direct bonding with an Er,Cr:YSGG hydrokinetic laser system.Am J Orthod Dentofacial Orthop 2002;122:649-56.
14. Harazaki M, Isshiki Y. Soft laser irradiation effects on pain reduction in orthodontic treatment. Bull Tokyo Dent Coll. 1997;38:291-295.
15. Fukui T, Harazaki M, Muraki K, Sakamoto T, Isshiki Y, Yamaguchi H. The evaluation of laser irradiated pain reductive effect by occlusal force measurement. Orthod Waves. 2002; 61:199-206.
16. Lim HM, Lew KK, Tay DK. A clinical investigation of the efficacy of low level laser therapy in reducing orthodontic postadjustment pain. Am J Orthod Dentofacial Orthop.1995;108:614-622.
17. Saito S, Mikikawa Y, Usui M, Mikawa M, Yamasaki K, Inoue T, Shibasaki Y. Clinical application of a pressure-sensitive occlusal sheet for tooth pain-time-dependent pain associated with a multi-bracket system and the inhibition of pain by laser irradiation. Orthod Waves. 2002;61:31-39.
18. Turhani D, Scheriau M, Kapral D, Benesch T, Jonke E, Bantleon HP. Pain relief by single low-level laser irradiation in orthodontic patients undergoing fixed appliance therapy. 377.
18. Mah J, Hatcher D. Current status and future needs in craniofacial imaging. Orthod Craniofac Res 2003;6 Suppl 1:10-6;179-82.
19. Quintero JC, Trosien A, Hatcher D, Kapila S. Craniofacial imaging in orthodontics: historical perspective, current status, and future developments. Angle Orthod 1999;69:491-506.
20. Kau CH, Zhurov AI, Bibb R, Hunter L, Richmond S. The investigation of the changing facial appearance of identical twins employing a three-dimensional imaging system. Orthod Craniofac Res 2005;8:85-90.
21. Yamada T, Mori Y, Katsuhiro M, Katsuaki M, Tsukamoto Y. Three-dimensional analysis of facial morphology in normal Japanese children as control data for cleft surgery. Cleft Palate Craniofac J 2002;39:517-26.
22. Nute SJ, Moss JP. Three-dimensional facial growth studied by optical surface scanning. J Orthod 2000;27:31-8.
23. Ayoub AF, Siebert P, Moos KF, Wray D, Urquhart C, Niblett TB. A vision-based three-dimensional capture system for maxillofacial assessment and surgical planning. Br J Oral Maxillofac Surg 1998;36:353-7.
24. Ayoub AF, Wray D, Moos KF, Siebert P, Jin J, Niblett TB, et al. Three-dimensional modeling for modern diagnosis and planning in maxillofacial surgery. Int J Adult Orthod Orthog Surg 1996; 11:225-33.
25. Ji Y, Zhang F, Schwartz J, Stile F, Lineaweaver WC. Assessment of facial tissue expansion with three-dimensional digitizer scanning. J Craniofac Surg 2002;13:687-92.
26. Khambay B, Nebel JC, Bowman J, Walker F, Hadley DM, Ayoub A. 3D stereophotogrammetric image superimposition onto 3D CT scan images: the future of orthognathic surgery. A pilot study. Int J Adult Orthod Orthog Surg 2002;17:331-41.
27. McCance AM, Moss JP, Fright WR, Linney AD, James DR. Three-dimensional analysis techniques-Part 1: three-dimensional softtissue analysis of 24 adult cleft palate patients following Le Fort I maxillary advancement: a preliminary report. Cleft Palate Craniofac J 1997;34:36-45.
28. Marmulla R, Hassfeld S, Luth T, Muhling J. Laser-scan-based navigation in cranio-maxillofacial surgery. J Craniomaxillofac Surg 2003;31:267-77.
29. McCance AM, Moss JP, Wright WR, Linney AD, James DR. A three-dimensional soft tissue analysis of 16 skeletal Class III patients following bimaxillary surgery. Br J Oral Maxillofac Surg 1992;30:221-32.
30. Moss JP, Ismail SF, Hennessy RJ. Three-dimensional assessment of treatment outcomes on the face. Orthod Craniofac Res 2003;6 Suppl 1:126-31; 179-82.
31. Ismail SF, Moss JP, Hennessy R. Three-dimensional assessment of the effects of extraction and nonextraction orthodontic treatment on the face. Am J Orthod Dentofacial Orthop 2002;121: 244-56.
32. McDonagh S, Moss JP, Goodwin P, Lee RT. A prospective optical surface scanning and cephalometric assessment of the effect of functional appliances on the soft tissues. Eur J Orthod 2001;23:115-26.
33. Wisth PJ, Nord A. Caries experience in orthodontically treated individuals. Angle Orthod 1977;47:59-64.
34. Gorelick L, Geiger A, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod 1982;81:93-8.
35. Årtun J, Brobakken BO. Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod 1986;8:229-34.
36. Ogaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop 1988;94:68-73.
37. Backer-Dirks O. Post-eruptive changes in dental enamel. J Dent Res 1966;45:503- 11.
38. Marcusson A, Norevall L-I, Persson M. White spot reduction when using glass ionomer cement for bonding in orthodontics: alongitudinal and comparative study. Eur J Orthod 1997;19:233-42.
39. Bjelkhagen H, Sundstro¨m F, Angmar-Månsson B, Ryde´n H. Early detection of enamel caries by the luminescence excited by visible laser light. Swed Dent J 1982;6:1-7.
40. Angmar-Månsson B, ten Bosch JJ. Optical methods for the detection and quantification of caries. Adv Dent Res 1987;1: 14-20.
41. De Josselin de Jong E, Hall AF, van der Veen MH. Quantitative light-induced fluorescence detection method: a Monte Carlo simulation model. In: Stookey GK, editor. Early detection of dental caries. Indianapolis: Indiana University, School of Dentistry; 1996. p. 91-103.
42. Ten Bosch JJ. Light scattering and related methods in caries diagnosis. In: Stookey GK, editor. Early detection of dental caries. Indianapolis: Indiana University, School of Dentistry; 1996. p. 81-90.
43. Kharsa MA. Use of laser in controlling the growth of facial structures, ?Laser-Orthopedics?. Orthod Cyberjournal August 2005.
44. Stenvik A, Mjrr IA. Pulp and dentin reactions to experimental tooth intrusion- a histological study of the initial changes. Am J Orthod 1970; 57: 370-385.
45. McDonald F, Pitt Ford TR. Blood flow changes in permanent maxillary canines during retraction. Eur J Orthod 1994; 16: 1-9.
46. Unterseher R, Nieburg L, Weimar A, Dyer J. The response of human pulpal tissue afterorthodontic force application. Am J Orthod Dentofacial Orthop I987; 92: 220-224.
47. Anstendig HS, Kronman JH. A histological study of pulpal reaction to orthodontic tooth movement in dogs. Am J Orthod 1974; 42: 50-55.
48. Spurrier SW, Hall SH, Joondeph DR, Shapiro PA, Reidel RA. A comparison of apical root resorption during orthodontic treatment in endodontically treated and vital teeth. Am J Orthod Dentofacial Orthop 1990; 97: 130-134.
49. Ztun J, Urbye KS. The effect of orthodontic treatment on periodontal bone support in patients with advanced loss of marginal periodontium. Am J Orthod Dentofacial Orthop 1988; 93: 143-148.
50. Barwick PJ, Ramsay DS. Effect of brief intrusive force blood flow on human pulpal blood flow. Am J Orthod Dentofacial Orthop 1996; 110: 273-279. |