Introduction
Periodontal diseases are the inflammatory diseases caused by microbial plaque accumulated on the tooth surface. The severity as well as clinical manifestations of the disease depends on the immune-inflammatory reaction manifested by host defense mechanism.
The production of cyclooxygenase products of arachadonic within periodontal tissues may partly mediate the destructive process of the periodontal tissues[1],[2]. The ability of the non-steroidal anti-inflammatory drugs (NSAIDS) to block cyclooxygenase pathway and reduce the prostaglandin synthesis led to series of studies demonstrating inhibition of periodontal disease progression.
Flurbiprofen is one of the most widely studied NSAID due to its potent inhibition of alveolar bone loss in periodontal disease[3],[4]. Although relatively low dose systemic NSAIDS have been successfully used in periodontics, it is not without side-effects. The major among these was gastrointestinal tract irritation. It is therefore desirable to formulate an agent that will deliver an effective dose of NSAID into periodontal tissues with minimum side effects. In general, topical application of NSAIDs is possible because these drugs are lipophilic and are absorbed into gingival tissues.
In this regard, safe and intrinsically efficacious medications can be delivered into periodontal sites to suppress or modulate the inflammatory host response. Different forms of controlled-release systems are like solutions, pastes, hollow fibers, acrylic strips, monolithic fibers, resorbable cellulose, collagen and biodegradable gel. Using controlled release gel from within the pocket, a single administration of few milligrams of a chemotherapeutic agent can maintain therapeutic concentrations within crevicular fluid for long time than other mode of delivery[5].
The present study was undertaken to study the sustained release of the drug(Flurbiprofen) from the delivery system in in vitro conditions as well as to study the Gingival Crevicular fluid(GCF) concentrations of the drug(Flurbiprofen) from the respective gel systems at various intervals.
Material and Methods
Flurbiprofen is a NSAID and is potent cyclooxygenase inhibitor. We have instituted this study to evaluate the effect of flurbiprofen on gingival inflammation when placed locally as controlled release drug delivery system. It was also very important to the study the concentration of drug released from the delivery system over the period of time in vitro and then the concentration of the drug in vivo.
There are number of human studies and trials using flurbiprofen. Hence the safety of the drug is well proven. The drug used here was in concentration of 0.3%, which was least likely to cause any systemic
 | Table 1: Ingredients Of In Situ Gel
 |
side effects. The study was reviewed and approved by the ethical committee of Kasturba Medical College, Manipal. The in vitro stud y was performed at College of Pharmaceutical Sciences, Manipal, India and the in vivo analysis was done at College of Dental Sciences, Manipal, India.
The ingredients of the gel are given in the [Table 1]. Accurately weighed amount of PLGA (copolymer ratio 75:25, having intrinsic viscosity 0.55 to 0.75dl/gm.) was placed in a glass vial and the required amount of biocompatible solvents was added. The vial was agitated using a mechanical shaker overnight to obtain clear solution. Weighed amount of drug was added to the polymer solution. Weighed amount of drug was added to the polymer solution, sonicated repeatedly to get uniform dispersion. The formulation contained 18% PLGA(75:25) in NMP, Flurbiprofen(15mg), PLGA (360mg), NMP(1.625gms).
The phosphate buffer saline was used to collect the gingival crevicular fluid samples for analysis of the drug levels.
In vitro evaluation of in situ gel
In vitro release studies of the drug delivery system is an important property of the characterization of the system. The in vitro drug estimation was performed in the simulated in vivo conditions so that the performance of the system in in vivo conditions can be predicted. The percentage cumulative drug release was estimated using UV spectrophotometer.
In vivo evaluation of drug concentration
The patients with localized periodontal pocket were selected for the study. Care was taken to ensure that patients do not have any systemic diseases, history of antibiotic or anti-inflammatory therapy in past 6 months. The patients who received gel were sub-divided into two categories viz., one with gel placement only (GO) and other with oral prophylaxis followed by gel placement(GOP).
The area of placement was dried properly and isolated. The pocket wall was then separated from the tooth surface with the help of an air syringe. 0.2ml of the gel was placed carefully in the pocket with the help of syringe and needle. When solution comes in contact with in vivo aqueous environment, it transforms into solid matrix that releases the loaded drug for prolonged period of time.
The gingival crevicular fluid was collected following gel placement and then at every recall visit i.e. on the 7 and 14 day after gel placement. Whatman No.1 filter paper discs of 6mm diameter were used to collect the samples. For collection of the samples the experimental tooth was cleaned and isolated. The filter paper disc was carefully placed into sulcus and left in the position for 3 minutes. Then it was removed and immediately transferred to a vial containing 5ml of isotonic phosphate buffer saline. The vial containing the sample was then closed and vigorously shaken for two minutes and then left undisturbed for 10 minutes. This was done so that the entire drug absorbed could be extracted from filter paper. At the end of 10 minutes this solution was filtered to remove any impurities like blood or plaque. The filtered solution was thus analyzed using UV spectrophotometer to obtain absorbance of the drug in 1ml of solution.
Results and Discussion
A sustained release delivery system consists of either non-resorbable or bioabsorbable matrices. They consist of a drug reservoir and a limiting element that controls the rate of medicament release. The present study was planned to develop a controlled release formulation of NSAID flurbiprofen to be used in the periodontal pocket as an adjunct to the conventional periodontal treatment.
As a first step, the PLGA copolymer in NMP containing 0.3% flurbiprofen was prepared. The in vitro release of the drug was then observed for 360 hours. The use of polymeric drug delivery devices in dentistry has added the new dimensions to the research in the field of locally
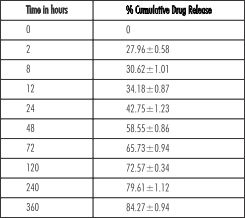 | Table 2: In Vitro Drug Estimation
 |
delivering the desired drug for the effective results. PLGA is a biodegradable copolymer, widely used as a vehicle for controlled release of different drugs[6] as well as growth hormones[7] in the medical research.
The in vitro percentage cumulative release of the drug from the delivery system was found to be 27.96±0.58 on the first estimation at 2 hours. The drug was steadily releases from the in situ gel system over the period of time to 84.27±0.94 on 15 day (Table 2).
Local drug delivery using NSAIDs have been evaluated in paste, gel form and mouth rinse form. The series on animal experiments have revealed the effectiveness of this form of drug delivery in periodontal diseases and stressed on the need for further research in this field[8],[9].
In this study flurbiprofen was selected to evaluate its host modulation effect in localized periodontitis. Various studies, utilized flurbiprofen in local delivery device in the form of toothpaste, local irrigation or in gel form[8],[9],[10]. Flurbiprofen is found to be a potent anti-inflammatory agent, showed significant decrease in alveolar bone loss as compared to other agents[11],[12].
The Delivery system used here was easy to dispense in the deep periodontal pockets. The gel solidifies as it comes in contact with aqueous environment and hence takes up the shape of the pocket. This helps in better retention of the delivery system without any need for the periodontal dressings.
In the in vivo study period was restricted to 2 weeks depending on the results of the in vitro study where the percentage
 | Table 3: Drug Concentration in GCF(µg/µl)
 |
cumulative drug release was calculated to be around 85% by 15th day. The similar rate of drug release was also found by Yewey et al (1991) in an animal experiment using flurbiprofen gel. The probing of the periodontal pocket for 2 weeks post-treatment is also not desirable.
In in vivo evaluation it was found that the mean drug concentration immediately after gel placement in GCF was 13.16±2.42µg/µl in GO group and 11.86±3.65µg/µl in GOP group. The drug concentration in GCF was eventually reduced to 6.87±1.32µg/µl in GO group on day 14, while the drug concentration was 6.20±2.34µg/µl.
It was also noticed that drug concentration decreased steadily in some of the subject on 7th and 14th day, while in some there was marginal increase in the drug concentration on day 14 as compared to day. The initial higher drug was probably due to the rapid release of drug from the fluid gel prior to its solidification within the periodontal pocket. (Table 3).
This study was aimed at the development of controlled-release formulations of flurbiprofen suitable for use in the periodontal pocket and to our knowledge first of its kind where flurbiprofen controlled release system in gel form is used in humans. If and when successful controlled-release formulations of anti-inflammatory agents are developed, the inflammatory cytokines and enzymes derived from the host cells can be effectively controlled, eventually controlling periodontal tissue destruction.
In conclusion, the delivery system devised in this study was able to release the required drug in a sustained manner over the period of the study both in vitro and in vivo without the reports of any kind of side-effects or discomfort to the patient. Such novel vehicles of the delivering the desired drug to the desired site with definitely take the host-modulatory therapies in periodontal diseases to new heights.
References
1. Goldhaber P, Rabadjija L, Beyar WR, Kornhauser A. Bone resorption in tissue culture and its relevance to Periodontal disease. J Am Dent Assoc 1973;87:1027-33
2. Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986 Mar;21(2):101-12.
3. Kurtis B, Tüter G, Serdar M, Pinar S, Demirel I, Toyman U. Gingival crevicular fluid prostaglandin E(2) and thiobarbituric acid reactive substance levels in smokers and non- smokers with chronic periodontitis following phase I periodontal therapy and adjunctive use of flurbiprofen. J Periodontol. 2007 Jan;78(1):104-11.
4. Salvi GE, Lang NP. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. Curr Pharm Des. 2005;11(14):1757-69.
5. Randive KS, Bhat KM. Local antimicrobial delivery in periodontal therapy: Indian J Dent Res 1988:4;124-30
6. Lim TY, Poh CK, Wang W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J Mater Sci Mater Med. 2009 Aug;20(8):1669-75.
7. Rafi M, Singh SM, Kanchan V, Anish CK, Panda AK. Controlled release of bioactive recombinant human growth hormone from PLGA microparticles. J Microencapsul.2010;27(6):552-60.
8. Heasman PA, Seymour RA, Boston PF. The effect of a topical non-steroidal anti-inflammatory drug on the development of experimental gingivitis in man. J Clin Periodontol. 1989 Jul;16(6):353-8.
9. Yewey GL, Tipton AJ, Dunn RL, Manardi EM, McEnvoy RM, et al. Evaluation of biodegradable sub gingival delivery system for Flurbiprofen. J Dent Res. 1991;70:324
10. Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid levels of interleukin-1 beta, leukotriene B4, pro staglandin E2, thromboxane B2 and tumour necrosis factor alpha in experimental gingivitis in humans. J Periodontal Res. 1993 Jul;28(4):241-7.
11. Jeffcoat MK, Williams RC, Wechter WJ, Johnson HG, Kaplan ML, Gandrup JS, Goldhaber P. Flurbiprofen treatment of periodontal disease in beagles. J Periodontal Res. 1986 Nov;21(6):624-33.
12. Williams RC, Jeffcoat MK, Kaplan ML, Goldhaber P, Johnson HG, Wechter WJ. Flurbiprofen: a potent inhibitor of alveolar bone resorption in beagles. Science. 1985 Feb 8;227(4687):640-2. |