Introduction:
Pleomorphic adenoma (PA) known as mixed tumor is the most common salivary gland neoplasm[1]. It was designated by terms like branchioma, enclavoma, teratoma, myxochondrocarcinoma, cylindroma, endothelioma, myxochondrosarcoma, chondromyxohemangioendothelioma, enchondroma[2]. The term Pleomorphic adenoma given by Willis, does not strictly imply cellular/ nuclear pleomorphism. Microscopically PA's are characterized by myriad of morphological diversity, composed of epithelial and myoepithelial cells arranged in variable patterns and demarcated from surrounding tissues by fibrous capsule. Three main histological subtypes are identified: 1) myxoid (stroma rich), 2) cellular (myoepithelial predominant) and 3) mixed (classic) type[3].
Case Report:
A 40yr female patient presented with a painless slow growing swelling on palate. The lesion was noticed 1 month back by the patient. Her medical history was noncontributory with no significant abnormalities on general physical examination. Patient underwent extraction of grossly decayed teeth two years back. Extraorally no abnormality was detected (Figure 1). Intraoral examination revealed a 3X3X3 cm swelling over hard palate just right to midline. (Figure 2). Anteroposteriorly, the swelling extended from the distal aspect of 14, posteriorly to the distal aspect of 18. Mediolaterally, the swelling extended from the mid - palatal area to the palatal surface of maxillary molar teeth. The mucosa over the swelling appeared to be normal with no secondary changes. Intraorally on palpation, the swelling was non - tender, firm in consistency, non- compressible and did not show any fluctuation or pus discharge. Patient had missing teeth in relation to 16, 26. Occlusal radiograph was taken and it revealed bony destruction (Figure 3). Provisional diagnosis of Pleomorphic adenoma was given, differential diagnosis included Adenoid Cystic Carcinoma, Mucoepidermoid Carcinoma, Adenocarcinoma, odontogenic and non-odontogenic cysts. Incisional biopsy was done. Histologically, the tumor mass showed glandular epithelial cells arranged in ductal pattern, eosinophilic material was present in the ducts (Figure 4, 5). Numerous myoepithelial cells arranged in the form of sheets were also seen. The supporting connective tissue stroma is moderately collagenous. Chondroid material is seen between cells (Figure 6). Hyalinized areas are seen in few areas (Figure 7). Inflammatory cell infiltrate is minimal Treatment included tumor excision with a wide margin.
 | Figure 1: Clinical picture showing no extraoral abnormality
 |
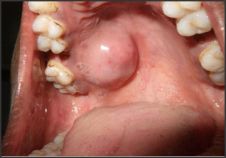 | Figure 2: Clinical picture showing intraoral swelling on hard palate
 |
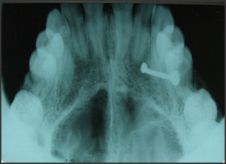 | Figure 3: Occlusal radiograph showing bone destruction
 |
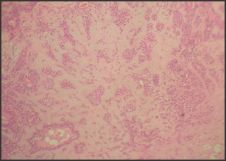 | Figure 4: Photomicrograph showing ductal and myoepithelial cells (H & E 10x)
 |
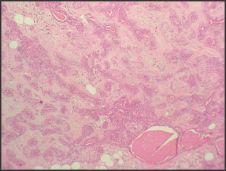 | Figure 5: Photomicrograph showing eosinophilic material in ducts (H & E 10x)
 |
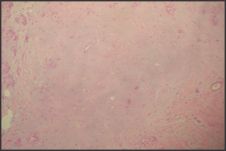 | Figure 6: Photomicrograph showing Chondroid areas (H & E 40x)
 |
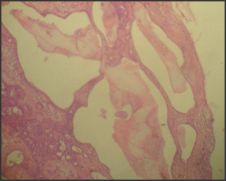 | Figure 7: Photomicrograph showing Hyalinized areas (H & E 40x)
 |
Discussion:
Pleomorphic adenomas over a wide age range, with peak incidence in fourth to sixth decade[4]. There is a slight female predilection. It commonly arise in parotid gland and may also occur in other major salivary glands, minor salivary glands, extra-salivary tissues like nasal cavity, paranasal sinus, larynx, bronchus, breast, soft tissues, ear, lacrimal gland and skin[5]. PA's are the most common salivary tumors showing synchronous[6] or metachronous[7] association with other salivary gland neoplasm, especially with Warthin's tumor. As the lesions are asymptomatic, there is a large interval between the first symptoms and the actual time of diagnosis ranging from 2 days to 15 years, with average time period of 25 months[8]. Pleomorphic adenoma clinically presents as a slow growing, painless, quiescent mass which slowly begins to increase in size, sometimes showing intermittent growth. They are round to oval in shape, showing lobulations when it increases in size. Recurrent tumors are multinodular. Size of the lesion varies from 2-6 cm in diameter1; few cases reported giant PA's measuring upto 28cm in size[9]. Smaller lesions have smooth surface, whereas the larger lesions show bosselations and is occasionally crossed by deep furrows. Fixation to either underlying or overlying skin is not seen, except in palatal tumors which are often fixed to mucoperiosteum. On palpation PA's are firm to rubbery with cystic degeneration occasionally if they are superficial. Overlying skin or mucosa is normal without ulcerations. Facial nerve palsy or pain may be seen in cases where the tumors have undergone infarction or become infected.
On gross examination, PA's appear as well circumscribed, round to ovoid mass with smooth or bosselated surface. There is variation in presence and thickness of capsule. They are often encapsulated and a clear demarcation between the tumor and adjacent salivary gland tissue is present. In minor gland PA, capsule is poorly formed or absent. Focal absence or complete absence of encapsulation with tumor merging into normal parotid gland is seen predominantly in mucoid tumors[10]. Recurrent lesions are multinodular and the nodules may bulge through capsule like pseudopodia[11]; satellite nodules are also reported[10]. Cut surface is solid and white with variable areas of firmer, translucent, bluish tissue. It may have gritty areas and gelatinous foci which correspond to cartilage like material seen microscopically. Necrosis and cystic degeneration may be seen in long standing cases.
Microscopically, PA has a biphasic appearance resulting from admixture of inner layer of epithelial cells which are large, cuboidal cells with vacuolated nuclei without prominent nucleoli, outer myoepithelial cells which have variably eosinophilic to clear cytoplasm and condensed, smaller, darker nuclei with flattened or triangular shaped cells in stromal background in a centrifugal or melting pattern in variable proportions[1],[2]. Foote and Frazell categorized them into principally myxoid, myxoid and cellular components in equal proportions, predominantly cellular, extremely cellular[12]. Siefert has classified based on the amount of extracellular stroma into Type 1: extracellular stroma comprises 30-50% of tumor (30% cases) Type 2: extracellular stroma comprises 80% of tumor (55% cases) Type 3: extracellular stroma comprises 20-30% of tumor (9% cases) Type 4: extracellular stroma attains a similar proportion to that of type 3 but there is focal monomorphic differentiation in the epithelial component[13].
Epithelial component shows cuboidal, basaloid, squamous cells arranged in the form of ducts, cords, cysts, islands, sheets, nests, trabeculae, tubules, ribbons. Rarely mucous, spindle, clear, oncocytoid, sebaceous, adipose cells, serous acinar cells are also seen. Ducts often contain eosinophilic secretory material. Myoepithelial cells are not readily identifiable by light microscopy as they may be small, dark and non descript, spindle shaped, plasmacytoid, polygonal, clear cells.[1],[2],[3].
Plasmacytoid cells are most prominent in PA especially in minor salivary glands[2] and strongly support diagnosis of PA. They are diffuse masses of round cells with hyalinized eosinophilic cytoplasm and eccentric nuclei that resemble plasma cell. Plump, eosinophilic staining, spindle shaped cells that form anastomosing bands or fascicles may be frequently seen after plasmacytoid cells[3]. Nuclear palisading may be present and the pattern may resemble leiomyoma or schwannoma however immunohistochemistry aids in differentiation. Cuboidal cells are the third most common cellular type[3], and the presence of this basaloid cells resemble basal cell adenoma. However, BCA's do not have Chondroid areas thus can be differentiated. Polygonal cells may have fine reticular arrangement. Epithelial islands with a cribriform structure are occasionally seen which resemble Adenoid cystic carcinoma (ACC). Presence of infiltration and perineural invasion, absence of squamous and chondroid metaplasia helps in differentiating fromACC from PA, Mucous goblet cells may be found in association with squamous cells and isolated foci may resemble Mucoepidermoid carcinoma. Absence of mucous metaplasia and presence of plasmacytoid cells and spindle cells help in differentiating PA from MEC. Foci of squamous metaplasia with keratin pearl formation may be seen[1]. Clear cells are seen occasionally which can be confused with that of Epithelial-myoepithelial carcinoma but clear cells in PA are small, hyperchromatic, angulate, whereas in EMC they are large and vesicular[1]. Occasionally tumors have areas of sebaceous metaplasia, mucinous and/ or mucoepidermoid like material[1]. Focal areas of oncocytes may be seen and also adipose cells[2]. Tumors that have lipomatous stromal component of 90% or more are called lipomatous PA's[1].
Myoepithelial cells produce mesenchymal component, which may be myxoid, cartilaginous, hyalinized, calcified. Myxoid is the predominant type where epithelial cells are widely separated, surrounded by mucoid material. True cartilage formation containing type II collagen and keratin sulphate is present as a result of extensive accumulation of mucoid material around individual myoepithelial cells, vacuolar degeneration of these cells occur subsequently. Eosinophilic hyaline material which shows propensity for malignant change[3] appears as foci within cellular masses or form bands that separate epithelial cells into either small nests or strands of cells. Bone may form by a process similar to endochondral ossification or directly by stromal osseous metaplasia rather than from ossification of preexisting chondroid stroma.[1],[2].
Crystalloid deposition may be seen in PA's. Tyrosine rich crystals occur in association with myoepithelial cells in both benign and malignant neoplasms; they are glossy, eosinophilic structureless masses surrounding central core, refractile and are seen arranged in petal shaped clusters. Collagen crystalloids found in benign neoplasms are more common and are non-refractile, needle shaped structures that are arranged radially or form stellate structures[14]. Oxalate crystals have also been reported in few cases[2]. Occasionally stromal amyloid or the corpora amylacea like condensations can be seen[1]. Areas of chronic inflammation particularly in subcapsular region are seen. More extensive inflammation and prominent central necrosis may be seen in long standing cases. Necrosis may be result of spontaneous infarction.[15] Elastic fibres are seen in long standing cases in form of thick, fluffy branching fibres; this fibrotic change/ scarring is frequent finding in malignant cases of PA's.
Immunohistochemistry can be performed to aid in the diagnosis of PA. Myoepithelial cells show positivity for Keratin, S100, GFAP, Actin, Vimentin, Calponin, Maspin, HHf-35, BMP, Aggrecan; whereas Ductal epithelial cells show Cytokeratin, Carcinoembryonic antigen, Epithelial membrane antigen, Antiepithelial membrane antigen, CAM 5.2.
Various histogenic concepts have been suggested for the development of PA's, relating to myoepithelial cell and a reserve cell in intercalated duct. Hubner et al[12] postulated that the myoepithelial cell is responsible for production of fibrous, mucinous, chondroid, osseous areas. Regezei and Batsakiset al[12] postulated that the intercalated duct reserve cell can differentiate into ductal and myoepithelial cells and later inturn can undergo mesenchymal metaplasia due to its smooth muscle like properties. Dardick et al[12] stated that a neoplastically altered epithelial cell is responsible for the PA histogenetically due to its potential for multidirectional differentiation. PA's have been shown consistent cytogenetic abnormalities chiefly involving the chromosome region 12q 13-15. The putative pleomorphic adenoma gene (PLAG1) has been mapped to chromosome 8q 12.[12].
Treatment of choice of PA's is superficial parotidectomy with preservation of facial nerve for tumors arising within parotid; for PA's in minor salivary gland wide local excision with the removal of periosteum or bone if they are involved.[4]. The same treatment modality was followed in the case reported in the present article.
Recurrence may be associated due to incomplete capsule, intraoperative tumor rupture in which tumor contents spill into operative field, tumors, with high mesenchymal content, particularly chondroid, myxoid stroma[16]. Malignant transformation is very uncommon and incidence ranges from 1.9-23.3%.[9].
Conclusion:
Pleomorphic adenoma presents the characteristic microscopic appearance of epithelial and myoepithelial cells in stromal background. However, certain variations from the usual may be found which are believed to be because of myoepithelial cells differentiation. Though diagnosis can be made because of this characteristic histological appearance, pathologist has to be aware of the variants of Pleomorphic adenoma which could lead to misdiagnosis.
References:
1. Douglas R. Gnepp. Diagnostic surgical pathology of head and neck. Second edition.
2. Ellis GL, Auclair PL. Tumors of salivary glands, atlas of tumor pathology. 3rd series. Fascicle 17. Washington, DC: Armed Forces Institute of Pathology, 1996.
3. Fabio Augusto Ito, Jacks Jorge, Pablo Agustín Vargas, Márcio Ajudarte Lopes. Histopathological findings of pleomorphic adenomas of the salivary glands. Med Oral Patol Oral Cir Bucal. 2009 Feb 1;14 (2):E57-61.
4. Sanjay Byakodi. Shivayogi Charanthimath. Santosh Hiremath. J.J. Kashalikar. Pleomorphic adenoma of palate: a case report. Int J Dent Case Reports 2011; 1(1): 36-40.
5. Yadavalli Guruprasad. Pleomorphic Adenoma of Hard Palate. Archives of dental surgery 2010 Vol 1, Issue 1;73-74.
6. Raphael M. Nagler, DMD, MSc, PhD,a,b and Dov Laufer, DMD,a Haifa, Israel. Synchronous pleomorphic adenomas of the major salivary glands- A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:735-7.
7. Sindeval Jose´ da Silva, Gabriel Tadeu Costa, Adalberto Caldeira Brant Filho, Paulo Roge´rio Faria, Adriano Mota Loyola. Metachronous bilateral pleomorphic adenoma of the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:333-8.
8. J. Jorge, F. R. Pires, F. A. Alves, D. E. C. Perez, L. P. Kowalski, M. A. Lopes, O. P. Almeida. Juvenile intraoral Pleomorphic adenoma: report of five cases and review of the literature. Int. J. Oral Maxillofac. Surg. 2002; 31: 273-275.
9. Ademar Takahama Jr, Danyel Elias da Cruz Perez, José Magrin, Oslei Paes de Almeida, Luiz Paulo Kowalski. Giant pleomorphic adenoma of the parotid gland. Med Oral Patol Oral Cir Bucal. 2008 Jan1;13(1):E58-60.
10. Andresa Borges Soares, Albina Altemani, Vera Cavalcanti de Araujo. Study of histopathological, morphological and immunohistochemical features of recurrent Pleomorphic adenoma: an attempt to predict recurrence of Pleomorphic adenoma. JOPM (2011)40: 352-358.
11. Korgun Koral, James Sayre, Sunita Bhuta, Elliot Abemayor, Robert Lufkin. Recurrent Pleomorphic Adenoma of the Parotid Gland in Pediatric: value of multiple lesions as a diagnostic indicator. AJR 2003; 180:1171-1174.
12. Shafer's textbook of oral pathology. 6th edition.
13. Steven G. Silverberg. Surgical pathology and cytopathology. 3rd edition.
14. Wallace G. Campbell, Robert E. Priest, Dwight R. Weathers. Characterization of two types of crystalloids in Pleomorphic adenomas of minor salivary glands. A Light-Microscopic, Electron-Microscopic, and Histochemical Study. Am J Pathol 1985,118: 194-202.
15. YK Chen, CC Lin, S Lai, CH Chen, WC Wang, YR Lin, SS Hsue, LM Lin. Pleomorphic adenoma with extensive necrosis: report of two cases. Oral Diseases (2004) 10, 54-59.
16. Peter Zbären, Isabelle Tschumi, Michel Nuyens, Edouard Stauffer. Recurrent pleomorphic adenoma of the parotid gland. Am jrnl of surg 189(2005) 203-207.
|