Introduction
Periodontal disease is a complex multifactorial disease characterized by destruction of periodontal tissues and loss of connective tissue attachment. Periodontal diseases are considered infections of the periodontium, because there is bacterial etiology. The microorganisms may exert pathogenic effects directly by causing tissue destruction or indirectly by stimulating and modulating host responses. The local alteration and destruction of host tissues as a result of microbial- host interactions may manifest as periodontal disease.[1]
The standard treatment of periodontitis consists of phase I periodontal therapy with the objective of reducing the total bacterial load and changing the environmental conditions of these microbial niches. Although mechanical treatment (scaling and root planing) reduces the level of subgingival bacteria, it does not eliminate all the pathogens which reside deep into the connective tissue and destroy the bone.
To overcome the limitations of this conventional treatment, antibiotics and antiseptics have been used successfully to treat moderate to severe periodontal disease by systemic and local administration. Systemic antibiotics require the administration of large dosages to obtain suitable concentrations at the site of disease, which potentially promote the development of bacterial resistance, drug interactions, and inconsistent patient compliance.
To overcome the shortcomings of systemic administration, a different approach has been introduced that uses local delivery systems that contain antibiotic or antiseptic agents. These systems allow the therapeutic agents to be delivered to the disease site with no appreciable systemic effects. Various locally delivered agents that are successfully used include tetracycline fibres[2], 10% doxycycline[3],[4], 2% minocycline[5], metronidazole[6] and chlorhexidine gluconate[7], but none are without side effects. To overcome the side effects of these drugs, research is being conducted for the use of the natural products. With the growing interest and increasing knowledge about the medicinal value of natural products, various formulations have been made commercially available. Natural products lead to a pathway of true and a healthy healing. One such natural plant which holds the medicinal value is Neem (Azadirachta indica).
Neem, a native tree of India is an incredible plant that has been declared the “Tree of the 21st century” by the United Nations. In India, it is variously known as “Divine Tree”, “Life giving tree”, “Nature’s Drugstore”, “Village Pharmacy” and “Panacea for all diseases”. The neem leaves, flowers, seeds, roots, bark and fruits are utilized to treat inflammation, infections, skin diseases and have been proved to be useful in dental care also. Neem has been used as the preferred tool to maintain healthy gums and teeth. It contains the alkaloid margosine, resins, gums, chloride, fluoride silica, sulphur, tannins, oils, flavenoids and calcium etc. Various other compounds like nimbin, nimbidin, ninbidol, sodium nimbidate and azadirachtin are also found in neem which act as anti-inflammatory, antipyretic and antihistaminic, antifungal, antimalarial, vasodilator, analgesic, antibacterial and antiulcer agents.[8] Neem oil suppresses various species of pathogenic bacteria such as Staphylococcus aureus and salmonella typhosa. The ethanolic extract has shown inhibitory effect on Escherichia Coli.[9],[10] Neem has been used in various forms such as neem twigs, mouthwashes and gels for the cure of gums. Based on the assumption of obtaining better efficacy of neem extract in the oral cavity when delivered in the form of indigeniously prepared chip, this clinical study was planned to evaluate the effect of neem chip when inserted into the periodontal pocket as an adjunct to SRP.
Materials and Method
30 otherwise healthy patients with chronic periodontitis in the age range of 35-55 years seeking dental treatment in the department of Periodontology of the institute were included in this study. All the patients were clinically diagnosed as having chronic periodontitis with at least one site presenting a pocket depth of
5mm bilaterally and presented with bleeding on probing.
This study was approved by the college ethical committee and the official permission was obtained from the Principal of the institution. Written informed consent was received from all the patients before their enrollment in the study. All the patients were in good health and had not received treatment with antibiotics/ anti-inflammatory drugs in last 3 months. Smokers and the patients with the history of systemic diseases were not included in the study. All patients were motivated and instructed for daily plaque control. Motivation for daily plaque control was reinforced at each visit. Education and motivation was followed by full mouth supragingival scaling using hand and ultrasonic scalers. Thorough subgingival scaling was done under local anesthesia in the selected sites using curets. 60 sites in total with 30 on each side (periodontal pockets) were selected and were divided into 2 groups.
Group I- It consisted of periodontal pockets (30 sites), in which only scaling and root planing (SRP) was done (control group).
Group II- It consisted of periodontal pockets (30 sites), in which scaling and root planing was followed by the placement of the neem chip inside the pocket (SRP+ Neem chip) (study group).
The day of completion of SRP was taken as baseline. Clinical parameters- Gingival index (Loe and Silness 1964)[11], Plaque index - Turskey et al modification of Quigley Hein index, probing pocket depth (PPD) and clinical attachment level (CAL) were recorded. The neem chip was inserted at the baseline in Group II. The patients were instructed to avoid eating sticky food for one week, to postpone brushing for 12 hours and to avoid using any mouth rinse or any irrigation device. The parameters were recorded again at 6 weeks and 3 months.
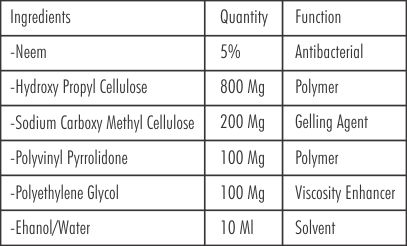
Neem chip for the study was indigenously prepared in the laboratory in Guru Nanak Institute of Pharmacy, Ibrahimpatnam, Hyderabad, under the supervision of Dr, S.A Sreenivas (Principal and Professor).
The composition of the neem chip along with the function is given as under.
 |
 |
Results & observations
All the patients did report for the two recall visits held at 6 weeks and 3 months. The data obtained was tabulated and analysed statistically.
The data was interpreted at a confidence interval of 95% and the levels of significance were as follows:
-P 0.05 - Not significant
-P 0.05 - Significant
-P 0.001 - Highly significant.
The mean probing pocket depth (mm) for group 1 (SRP) at baseline, 6 weeks and at 3 months were (5.40± 0.77 mm, 3.97± 0.67 mm, 3.33± 0.88 mm) respectively. The mean probing depth scores for group 2 (SRP+ Neem chip) at Baseline, 6 weeks, and at 3 months were (5.60± 0.81mm, 3.67± 0.96 mm, 3.20± 0.89 mm) respectively. (Table-I)
The mean gingival score for group 1 (SRP) at baseline, 6 weeks and at 3 months were (1.84± 0.19, 1.38± 0.38, 1.37± 0.36) respectively. The mean gingival score for group 2 (SRP+ Neem chip) at baseline, 6 weeks and at 3 months were (1.87± 0.21, 1.49± 0.39, 1.19 ± 0.30) respectively. (Table-II)
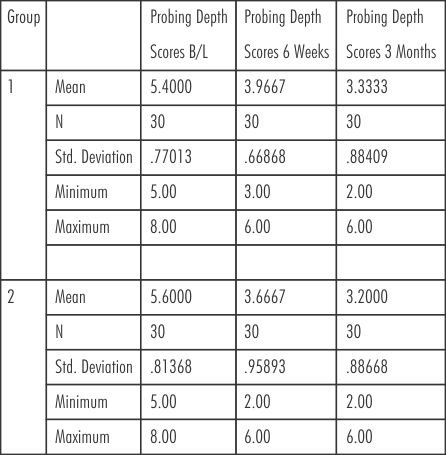 | Table - I Probing Pocket Depth
 |
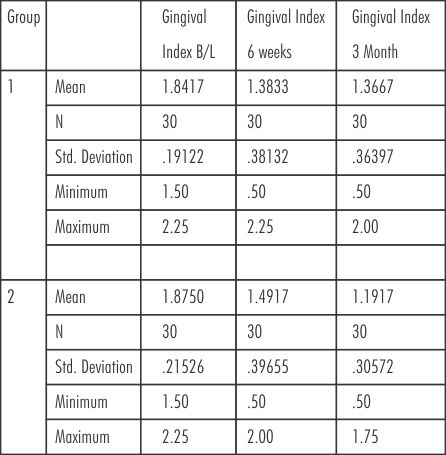 | Table- II Gingival Index
 |
Plaque scores were brought to minimum at baseline. The mean plaque scores for group 1(SRP) at baseline, 6 weeks and at 3 months were (0.00 ± 0.00, 3.02 ± 0.59, 3.22± 0.57) respectively. The mean plaque scores for group 2 (SRP+ Neem chip) at baseline. 6weeks and at 3 months were (0.00± 0.00, 2.93 ± 0.67, 3.20± 0.57) respectively. (Table-III)
The mean CAL score (mm) for group 1(SRP) at baseline, 6 weeks and at 3 months were ( 4.23± 0.57 mm, 3.97± 0.56 mm, 3.47± 0.63 mm) respectively. The mean CAL score for group 2 (SRP+ Neem chip) at baseline, 6 weeks and at 3 months were (4.23 ± 0.57 mm, 4.13± 0.63 mm, 3.57 ± 0.68 mm) respectively. (Table-IV).
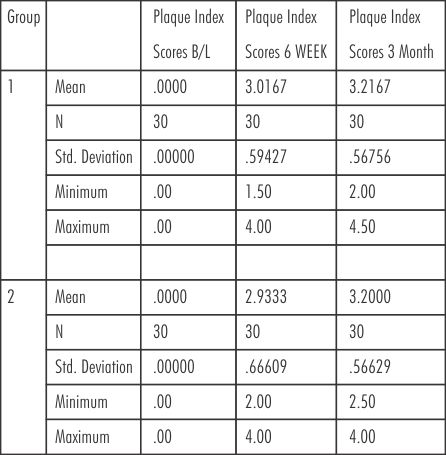 | Table - III Plaque Index Scores
 |
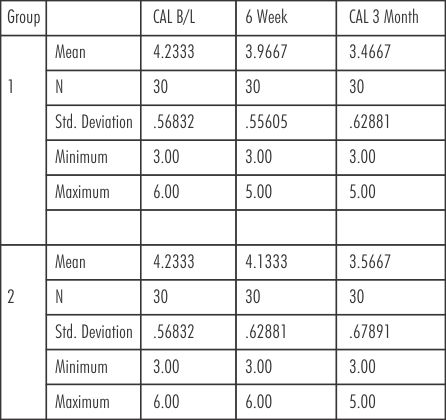 | Table - IV Clinical Attachment Level (CAL)
 |
For the comparison of the all the parameters between the two groups from baseline to 3 months t- test for equality of means (independent samples test) was applied. The findings showed statistically significant reduction in probing depth in group 2 (SRP+Neem chip) as compared to group 1(SRP) from baseline to 6 weeks (p = 0.001) and from baseline to 3 months (p= 0.045). The results were not statistically siginificant during changes observed from 6 weeks to 3 months (p= 0.257). (Table-V) .The results showed that there was reduction in the gingival scores in both the groups but were not statistically significant at 6 weeks (p= 0.285) whereas the results were statistically significant at 3rd month evaluation showing that there was reduction in the gingival scores significantly in group 2(SRP+Neem chip) (p= 0.048). (Table-VI)
There was accumulation of plaque in both the groups at 6 weeks and 3 months but the plaque scores were comparable for both the groups, showing that the results were not statistically significant. (p= 0.61and p= 0.91 at 6 weeks and 3 months respectively) (Table- VII)
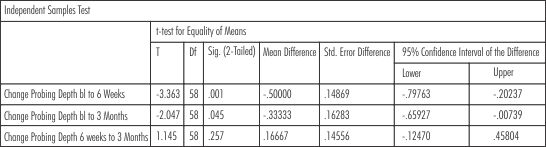 | Table -V Probing Pocket Depth (Intergroup Comparison)
 |
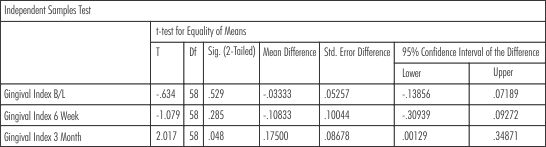 | Table - VI Gingival Index (Intergroup Comparison)
 |
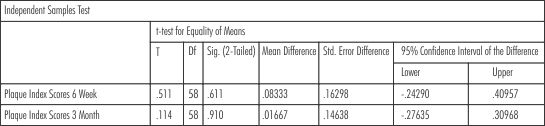 | Table - VII Plaque scores (Intergroup Comparison)
 |
There was a gain in the clinical attachment level in both the groups from baseline to 3 months but the scores were comparable, showing no statistically significant results.The p value at baseline, 6 weeks and 3 months was1.00, 0.28 and 0.56 respectively. (Table-VIII)
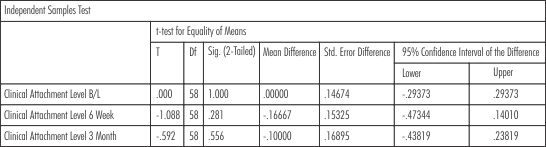 | Table - VIII Clinical Attachment Level (CAL) (Intergroup Comparison)
 |
Discussion
This clinical study evaluated the effectiveness of natural product 'neem', indigeniously prepared in the form of neem chips. Neem has been long considered to have an antiseptic property.The antibacterial activity of neem has been evaluated and known from ancient times.[12] A study by Rao et al (1986) has proved its efficacy in reducing plaque cultures and gram-negative bacteria compared to the commercially available dentrifice.[13] The results of present study supported the efficacy of neem in treating the periodontal disease also. In order to avoid the bitter taste of the various neem formulations, the neem chip was prepared for this study.
The clinical evaluation of the neem chip showed a statistical significant reduction in probing pocket depth (PPD) at 6 weeks and 3 months, and a significant reduction in gingival index score was also found at 3 month as compared to SRP alone. There was decrease in the plaque index scores and gain in the clinical attachment levels and the results were comparable in both the groups at 6 weeks and 3 month evaluation. The results may be attributed to various chemical compounds present in Neem such Nimbin; Nimbidin- anti-inflammatory, anti-bacterial, anti-fungal; Gedunin- vasodilator, anti-malarial, anti-fungal; Quercetin-anti-protozoal; Salannin- insectrepellent; Azadirachtin- anti-malarial.[14] The neem chip had an additive effect when used as an adjunct to scaling and root planing. The greater improvement in the clinical parameters and relatively lesser plaque accumulation in the SRP + neem group can be due to the adjunctive effect of the antimicrobial property of the slow released drug from the chip.The complete concentration of neem was released in 2 days, maintaining a concentration of 21.12 mcg/ml at 48 hrs post insertion in the subgingival environment. Carbopol was used as a gelling polymer due to its mucoadhesive property.
The antibacterial activity of neem has already been evaluated since ancient times.[15] The leaf extract of the neem has shown superior antiviral and antihyperglycemic activity in vitro and in vivo on animals.[16] Leaf extract has also shown hepatoprotective activity.[17]
A study was conducted by M. Raveendra Pai et al in 2003, evaluating effectiveness of neem leaf extract in the form of mucoadhesive dental gel comparing it with commercially available chlorhexidine gluconate mouthwash. They found that the patients (test group) using dental gel containing neem showed significant reduction of plaque index and a decrease in salivary bacterial count of streptococcus mutans and lactobacillus than the ones using chlorhexidine (control group).[18] Another study by Prashant GM et al in 2007 showed the antibacterial effect of neem and mango on streptococcus mitis.[19]
Neem showed better efficacy in reducing the human plaque cultures and gram-negative bacteria compared to the commercially available dentifrice. It has been reported to show minimal to almost no adverse effects. The patients in this study did not report with any complaints of allergy or discomfort due to the chip. This study indicates the use of neem in treating the periodontal diseases due to its antimicrobial effects as claimed by the traditional medicine. It concludes that the Neem chip formulated with a mucoadhesive polymer can significantly improve the periodontal conditions when used as adjunct to scaling and root planing. To prove it more beneficial further research is required for the formulation of more sustained drug release products using neem so that the drug is maintained at the site for a longer duration or by the insertion of the neem chip for the second time. The neem chip had a drug release for 2-4 days as compared to chlorhexidine chip which has 7 days.[7],[20] The results obtained present a valid premise for further long term studies with a larger sample size and microbiological parameters to evaluate the efficacy of neem chip for the management of chronic periodontitis. Thus inclining us to treat the diseases with natural products in order to avoid the side effects of other antimicrobial agents used locally. Thus making us believe in being natural and buying natural to be the pathway to true cure and healing.
Conclusion
This study establishes the use of natural product neem in the form of neem chip as an adjunct to scaling and root planing for treating the periodontal pockets. Neem chip formulated with a mucoadhesive polymer have additional benefits due to its antimicrobial property.
Refrences
1. Newman MG, Takei HH, Klokkevold PR and Carranza KA. Carranza's clinical periodontology. Tenth edition. Page- 228.
2. Goodson JM and Tanner A. Antibiotic resistance of the subgingival microbiota following local tetracycline therapy, Oral Microbiology and Immunology 1992; 7:113-117.
3. Larsen T: Occurrence of doxycycline resistant bacteria in the oral cavity after local administration of doxycycline in patients with periodontal disease. Scandinavian Journal of Infectious Diseases 1991; 23: 89-95.
4. Javali MA and Vandana KL. A comparative evaluation of atrigel delivery system (10% doxycycline hyclate) Atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J Indian Soc Periodontol 2012;16:43-8.
5. Steenberghe VD, Bercy P, Kohl J. Subgingival minocycline hydrochloride ointment in moderate to severe chronic adult periodontitis: a random, double blind, vehicle-controlled, multicenter study, J Periodontol 1993; 64: 637.
6. Stelzel M and Jacoby FDL: Topical metronidazole apllication compared with subgingival scaling: a clinical and microbiological study on recall patients, J Clin Periodontol 1996; 23 :24.
7. Soskolne WA, Heasman PA, Stabholz A, Smart GJ, Palmer M, Flashner M, Newman HN. Sustained local delivery of chlorhexidine in the treatment of periodontitis: a multicenter study, J Periodontol 1997; 68: 32.
8. Sharma P, Tomar L, Bachwani M, Bansal VM. Review of Neem (Azadirachta Indica): Thousand problems one solution. International Research Journal of Pharmacy 2011; 2: 97-102.
9. Singh B, Jindal N, Bansal R, Kumar D, Gupta V. Antimicrobial potential of polyherbomineral formulation Jatyadi Taila- A review. International Journal of Research in Ayurveda and Pharmacy 2011; 2: 151-156.
10. Jahan T, Begum ZA, Sultana S. Effect of neem oil on some pathogenic bacteria. Bangladesh Journal of Pharmacology 2007; 2: 71-72.
11. Silness J and Loe H. Periodontal disease index. Ann Periodontol. 1964;4:655-69.
12. Chatterjee A, Saluja M, Singh N, Kandwal A. To evaluate the antigingivitis and antipalque effect of an Azadirachta indica (neem) mouthrinse on plaque induced gingivitis: A double-blind, randomized, controlled trial. J Indian Soc Periodontol. 2011; 15: 398-401.
13. Rao DVK, Singh I, Chopra PC, Chabra, Ramanujalu G. In vitro antibacterial activity of neem oil. Indian Journal of Medical Research 1986;84:314-316.
14. Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica). Current science 2002; 82: 1336- 1345.
15. Chatterjee A, Saluja M, Singh N, Kandwal A. To evaluate the antigingivitis and antipalque effect of an Azadirachta indica (neem) mouthrinse on plaque induced gingivitis: A double-blind, randomized, controlled trial. J Indian Soc Periodontol 2011;15:398-401.
16. Chattopadhyay RR. Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract: PartV. Journal of Ethnopharmacology 1999; 67: 373-376.
17. Chattopadhyay RR. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. Journal of Ethnopharmacology 2003; 89: 217-219.
18. Pai MR, Acharya LD, Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel- a 6 week clinical study. J Ethnopharmacol 2004; 90: 99-103.
19. Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivarius, Streptococcus mitis and Streptoccocus sanguis: An in vitro study. Indian journal of Dental Research 2007;18:148- 151.
20. Soskolne WA, Chajek T, Flashner M, Landau I, Stabholtz A, Kolatch B, Lerner EI. An in vivo study of the chlorhexidine release profile of the periochip in the gingival crevicular fluid, plasma and urine. Journal of Clinical Periodontogy 1998;25:1017-1021. |