Introduction
Periodontal disease is a chronic microbial infection that triggers inflammation leading to loss of periodontal ligament and alveolar bone that supports the teeth. It is known that the periodontitis has an episodic nature that is period of activity and inactivity[1],[2]. Conventional diagnostic methods that is Bleeding on probing, Probing pocket depth, Attachment loss or gain and assessment of alveolar bone destruction cannot discriminate between disease activity and inactivity. These methods can detect only the past tissue destruction [3],[4]. So, new diagnostic techniques need to be developed to detect both active and inactive sites.
Cultural test that involve a quantitative anaerobic methods are considered the best means of enumerating actual number of colony forming units (CFU's) present in plaque. But the drawback is uncultured Spirochetes remains undetected. Dark or phase contrast microscopy has its ability to detect motile bacteria and spirochetes. But most of main putative periodontopathogens including Aggregatibacter actinomy cetemcomitans, Porphyromonas gingivalis, Prevotella intermedius, Eikenella corrodens and Eubacterium species are non motile and this technique is not able to identify these species. Other methods are Substraction radiography, Nuclear medicine techniques, Computerized tomography, DNA probing.
BAPNA hydrolysis test is designed to tackle the synthesis of tripsinoid enzyme produced by 3 pathogens:- P.gingivalis, T.forsythia and T.denticola [5],[6]. This enzyme not only degrades the extracellular matrix proteins of the host, it is also capable of hydrolyzing the Benzoyl argenine P-nitroanilide and Benzoyl DL-argenine napthylamide. Several Capnocytophaga and non pigmented Bacteroids species are variable in giving a weak BAPNA reaction. Fifty one other plaque species are not able to hydrolyse BAPNA and BANA. BANA hydrolysis will detect Porphyromonas gingivalis and Treponema denticola with a sensitivity of 92% and specificity of 70% with highly specific polyclonal antibodies to these organisms in an ELISA assay being used as the reference standard. Early detection of periodontal disease using BAPNA hydrolysis provides rationale for implementing treatment and allows the operator to intercept the disease at a primary level. The testing of innocuous sites in healthy and diseased individuals would help to clarify their true nature at a cellular level and expose vulnerable areas. This quality would also indirectly enable the clinician to monitor sites for the development of active periodontal disease and even predict future attachment loss. Chair side microbial and enzyme diagnosis are simple rapid and reliable in office tests. So, it was thought to carry out a controlled study with following objectives:-
1) To correlate between clinical parameters and BAPNA in periodontal disease.
2) To test utility of BAPNA test in clinical trial of efficacy of Metronidazole with and without scaling and root planing.
Materials And Methods
The present clinical study included 15 subjects aged between 35 and 65 years of age irrespective of sex were selected.1607 pockets were observed for the baseline recording of two parameters- BAPNA test and Probing pocket depth. In each patient the maxillary and mandibular arch of any one side, either right or left, was scaled and root planed. and patients were given 400mg Metronidazole tablet three times a day for 7 days and evaluation done after 14 days
Patient's Site Category
Group A - Subjects on Metronidazole therapy only.
Group B - Subjects with combined Metronidazole plus scaling and root planing.
Inclusion Criteria
15 patients were selected randomly of age 35-65 years, exhibiting chronic periodontitis who met the following selection criteria
1) Patient who complaint of bleeding during brushing of teeth or chewing of food or both, at least for past one week.
2) Patient with ten or more than ten natural teeth with clinical evidence of periodontal disease that is deep pocket associated with bleeding on probing were included in the study.
3) Individuals without any history of systemic disease.
4) Patient without recent history of antibiotic therapy in the last 3 months.
Probing Pocket Depth:
Pocket measurements were taken with William's periodontal probe. They were measured at four sites around each tooth that is mesio-buccal, mid buccal/facial, disto-buccal, mid lingual/palatal. Pocket depths that were measured are classified into 3 ranges: 1-3 mm, 4-6mm, 7mm & more..
Armamentarium:
Working solution- 0.174gm BAPNA was dissolved in 5 ml dimethylsulfoxide. 50 ml of trihydrochloride buffer, PH 8.2 and 10 ml of calcium chloride was added to it make the final volume of 100 ml.
Principle behind BAPNA test:
When the plaque sample was made to react with the substrate, i.e Benzoyl arginine P-nitroanilide (BAPNA), enzyme produced by the bacteria i.e trypsin acts on the BAPNA reagent producing benzoyl argenine and nitroanilide.
Benzyol arginine p-nitroanilide + water -> Benzoyl arginine +nitroaniline
With the amount of P-nitroaniline released, the colour of the solution changes from colourless to deep yellow. The intensity of yellow colour is directly proportional to the trypsin activity. The working solution is prepared from the stock solution as the diluted trypsin reaction mixture precipitate if kept for more than the required time of 10 days in the refrigerator. So, it is freshly prepared every 10 days. Erlanger described the properties and preparation of BAPNA solution in the similar way[7].
The ability to detect BAPNA hydrolyzing activity in subgingival plaque appears to be a simple & inexpensive means of monitoring periodontopathic bacteria like T. denticola, P. gingivalis & B. forsythus.
Plaque Sample Collection:
Subgingival plaque sample was collected with sterilized curettes (Colombia curettes 2R-2L, 4R-4L). This sample was immediately transferred to microtiter plate containing 0.1 ml of BAPNA solution. Plaque sample from the mesial surface of upper right central incisor was transferred to microtiter well labelled A1. Similarly, plaque sample from mid facial area of upper right central incisor was taken to the microtiter well labelled A2, A3 and A4 contained plaque sample from disto-buccal and palatal surface of upper right central incisor. Every fifth well of microtiter plate contained only the BAPNA working solution and not the plaque sample. This would act as a control for a colour change that were taking place in right and left wells.
Enzymatic Procedures:
The sample in BAPNA working solution was incubated for 24 hours at 370C[8],[9]. The colour change was read by naked eye. Similar criteria that was used for the colour change in BANA test (Loesche 1987) was utilized in the present study for BAPNA test. The results obtained for BAPNA were categorized as:
0-No colour change (absence of Treponema denticola, Porphyromonas gingivalis and Bacteroid forsythus)
1-Light yellow (minimum concentration of Treponema denticola, Porphyromonas gingivalis and Bacteroid forsythus)
2-Yellow (medium concentration of Treponema denticola, Porphyromonas gingivalis and Bacteroid forsythus)
3-Deep yellow (maximum concentration of Treponema denticola, Porphyromonas gingivalis and Bacteroid forsythus)
On an average the time taken for the recording was about 30 minutes.
Results
In the present study 15 patients were selected and 1607 pockets were observed for the baseline recording of the 2 parameters- BAPNA test and probing pocket depth.
Group A - 727 pockets at baseline received only Metronidazole therapy.
Group B - 880 pockets at baseline and received Metronidazole plus SRP.
Statistical analysis was done in which
Group A and B after treatment compared with baseline and Group A and B compared to each other after treatment as baseline in both groups were similar.
In BAPNA score ‘0’ maximum distribution in pocket depth of 1-3 mm is 86.02% in Group A (baseline) and 84.02% in Group B (baseline). In BAPNA score ‘1’ maximum distribution in pocket depth of 1-3 mm is 13.90% in Group A (baseline) and 15.64% in Group B (baseline). BAPNA score ‘2’ is absent at baseline (Table-1,3).
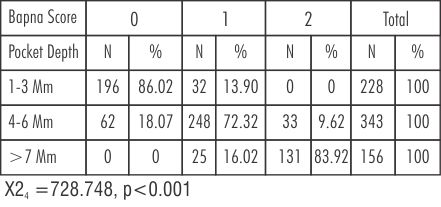 | Table 1: Percentage distribution of pocket depth with different BAPNA test scores in Group A at baseline ( non scaled sites without Metronidazole therapy)
 |
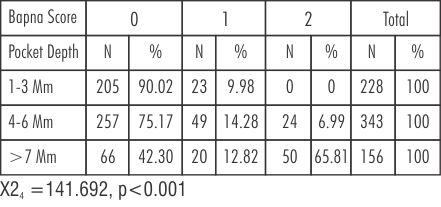 | Table 2: Percentage distribution of pocket depths in different BAPNA test scores in Group A after Metronidazole therapy
 |
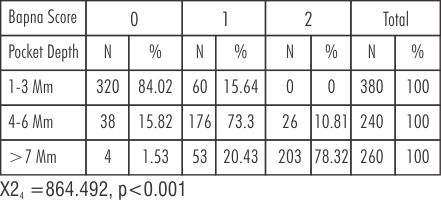 | Table 3: Percentage distribution of pocket depth with different BAPNA test scores in Group B at baseline
 |
In pocket depth of 4-6 mm maximum percentage of BAPNA score '1' is 72.32% and 73.30%. BAPNA score '0' is 18.07% and 15.82%. BAPNA score '2' is 9.62% and 10.81% in Group A and B at baseline respectively (Table-1,3).
In pocket depth >7mm BAPNA score '2' is 83.92% and 78.32%. BAPNA score '1' is 16.02% and 20.43%. BAPNA score '0' is 0% and 1.53% in Group A and B respectively at baseline (Table-1,3).
In pockets of 1-3mm comparing Group A at baseline and after Metronidazole therapy BAPNA score '0' percentage increases from 86.02% to 90.02%. BAPNA score '1' percentage decreases from 13.90% to 9.98% (Table-1,2).
In Pockets of 4-6mm, there was significant decrease in BAPNA score' 1' from 72.32% to 14.28%. BAPNA score '0' increases from 18.07% to 75.17%. BAPNA score' 2' decreases from 9.62% to 6.99%. In pockets of >7mm, BAPNA score '1' decreases from 16.02% to 12.82%. BAPNA score '0' increases from 0% to 42.30%. BAPNA score '2' decreases from 83.92 %to 65.81%
Comparing Group B at baseline and after Metronidazole +SRP (Table-3,4).
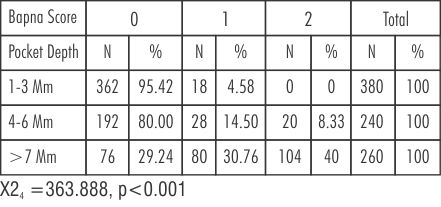 | Table 4: Percentage distribution of pocket depth with different BAPNA test scores in Group B after treatment (scaled sites with metronidazole therapy))
 |
Pockets of 1-3mm, BAPNA score '0' changes from 84.02% to 95.42%. BAPNA score '1' changes from 15.64% to 4.58%. BAPNA score '2' remains same from 0% to 0%. In pockets 4-6mm, BAPNA score' 0' increases from 15.82% to 80.00%. BAPNA score '1' decreases from 73.3% to14.50%. BAPNA score '2' decreases from 10.8% to 8.33%. In pockets of >7mm, BAPNA score '0' increases from 1.53% to 29.24%. BAPNA score '1' increases from 20.43 to 30.76%. BAPNA score' 2' decreases from 78.32% to 40.0%
(Table-1,2 and 5) depicts that in pocket of 4-6mm there is a significant decrease in BAPNA score '1' from 72.32% to 14.28% in Group A, showing statistically significant (P<0.001)values. The percentage of BAPNA score'0' increases from 18.07% to 75.17% in Group A.
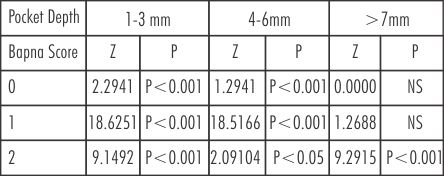 | Table 5: Comparison of BAPNA score in different pockets depths in group A at baseline and after treatment
 |
This increase was statistically significant (P<0.001). BAPNA score '2' from 9.62% to 6.99% after Metronidazole therapy alone there has been decreased in BAPNA positive periodontopathic bacteria.
(Table-3, 4 and 6) BAPNA score' 0' is higher in group B after treatment than baseline(80% to 15.82%) showing statistically significant change. Score '1' is increased significantly from 73.3% to 14.50% in 4-6mm, but there is no appreciable change in score '2'.
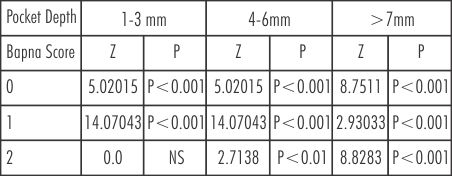 | Table 6: Comparison of BAPNA score in different pockets depths in Group B at baseline and after treatment
 |
When two different treatment modalities were compared it is observed that the percentage increased in BAPNA score '0' is higher in Group B than Group A.
In (Table-1 & 2), in pocket >7mm the percentage of BAPNA score '2' decreases from 83.92% to 65.81% in Metronidazole therapy while it is 78.32% to 40% in Metronidazole plus scaling root planing.
(Table 7) shows the comparison of BAPNA score and pocket depth in Group A and Group B after treatment.
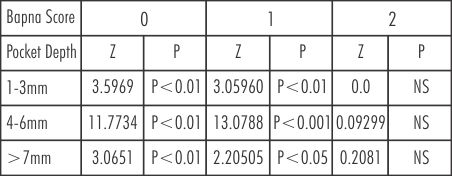 | Table 7: Change of BAPNA score with different pocket depths in Group A and B at baseline and after treatment
 |
Discussion
Diagnostic tests should be such that it is cost effective, rapid, useful and ideally leading to a Choice of treatment that would be beneficial to the patient. One such diagnostic aid is BAPNA test that not only fulfils all the above requirements but also is hydrolysed by a Trypsin like enzyme released by the putative pathogens like Treponema denticola, Porphyromonas gingivalis and Bacteroides forsythus which are mostly present in all forms of periodontal diseases.
Bretz reported that there was no difference in hydrolysis of BANA and BAPNA substrates and both of them gave similar results[10].Tsustui (1987) isolated the trypsin like enzyme from Bacteroides gingivalis which hydrolysed BAPNA[11].
In the present study 15 patients were selected and 1607 pockets were observed for the baseline recording of the 2 parameters-BAPNA test and probing pocket depth. In each patient the maxillary and mandibular arch on any one side either right or left was scaled and root planed.The patients were then given oral metronidazole therapy which showed its effect on both the arches while the other was local that is scaling and root planning,which was done in only two selected quadrants. So each patient received 2 different lines of treatment. Two quadrants received only metronidazole therapy and the other 2 quadrants received combined metronidazole and scaling and root planing.
In pockets of 1-3 mm BAPNA score is negative in maximum number of pockets in score '0'. That means absence of T. denticola, P. gingivalis & B. forsythus. The results of our study are in harmony with the result of Loesche et al (1987) who in their study determined the relationship of the BAPNA / BANA test and probing pocket depth.They found that sites with pocket depth of 2 or 3 mm had significantly lower proportion of Spirochetes and only 10% gave BANA / BAPNA positive reactions[12].
In pockets of 4-6 mm show weak BAPNA positive reaction that is minimum concentration of T. denticola, P. gingivalis, B. forsythus.The result are in accordance with the study of Loesche et al (1987) who showed that probing depth of 4 mm had 16% Spirochetes but were also associated with 26% BANA / BAPNA positive reactions and half of them were of weakly positive type. 5-6mm deep sites exhibited a significant increase in the proportion of spirochetes but the average value of 30% Spirochetes was associated with 52-57% of positive BANA / BAPNA reactions with the majority being of the positive type (score 2)[12] . In pockets of >7 mm range maximum pockets with positive BAPNA score i.e maximum concentration of T. denticola, P. gingivalis & B . forsythus.
After metronidazole therapy in pockets of range 1-3 mm, 4-6 mm and >7 mm there is increase in BAPNA negative sites. There is also increase in BAPNA negative sites treated with scaling and root planing. The BAPNA negative sites are much more in metronidazole plus SRP as compared to metronidazole alone but in >7mm there is not much change in two groups[13]. This could be explained by the fact that at pockets of greater than 6 mm, the efficacy of mechanical debridement decreases due to instrument inaccessibility of deep root surfaces and furcations.These sites however, are apparently bathed by sufficient levels of Metronidazole to effect a significant reduction in BAPNA positive periodontopathic bacteria.
So, it has been shown that BAPNA positive sites are converted to BAPNA negative sites which reduces the need of surgical intervention. This is dependent on host factors like host immune responses or maintenance of oral hygiene by patient at optimum level. These factors determine the tooth's sites specific response to a certain extent. The important utilization of BAPNA test would be its ability to diagnose anaerobic infection which enables the clinician to prescribe Metronidazole as an adjunct to SRP. The most important property of BAPNA test is that it indicates future attachment loss. Positive BAPNA test following active initial therapy supports this statement.
Conclusion
BAPNA hydrolysis by the plaque bacteria that is P.gingivalis, T. denticola, B. forsythus utilized for the management of anaerobic infections in periodontal disease. BAPNA test forms a basic forms a basis for the diagnosis of anaerobic infection. So, BAPNA test is reliable, easy & predict future attachment loss indicating a helpful technique for management of periodontally diseased patients.
References
1) Hirshfeld L, Wasserman. A long term survey of tooth loss in 600 treated patients. J Periodontal 1978; 49: 225-37.
2) Lindhe J, Haffajee AD, Socransky SS. Progression of periodontal disease in adult subjects in absence of periodontal therapy. J Clin Periodontal 1983; 10: 433-42.
3) Lindhe J, Okamoto H, Yoneyama T , Haffajee A , Socransky SS. Longitudinal changes in periodontal disease in untreated subjects. J Clin Periodontal 1989; 16: 662-70.
4) Lindhe J, Okamoto H, Yoneyama T, Haffajee A, Socransky SS. Periodontal loser sites in untreated adult periodontitis. J Clin Periodontal 1989; 16: 671-8.
5) Pederson ED, Miller J W, Matheson SS, Chadwick DE. Trypsin like activity levels of T.denticola and P.gingivalis in adults with periodontitis. J Clin Periodontology 1994;8(21): 519-25.
6) Ramfjord SP, Caffesse RG, Morrison HC. 4 modalities of periodontal treatment compared over 5 years. J. Periodontal 1987;14(8): 445-52.
7) Erlanger et al. The preparation and properties of trypsin. In Archieves of Biochemistry and Biophysics 1961; 95: 271-78.
8) Feitosa AC, Amalfitano J, Loesche WJ: The effect of incubation temperature on the specificity of the BANA test J; Oral microbial. Immunology 1993; 8(1): 57-61.
9) Joseph Amalfitano, Anna B, Loesche WJ: The effects of incubation length and temperature on the specificity and sensitivity of the N-Benzoyl-DL-arginine naphthyalamide (BANA) J Periodontal 1993;64:848-52.
10) Bretz WA and loesche WJ. Characteristics of trypsin like activity in subgingival plaque samples. In: J. Dent Res. 1987 Nov : 66 (11): 1668-72.
11) Tsutsui H et al. Purification and characterization of a protease from Bacteroides gingivalis 381.In: Infectious Immunity. 1987, feb;55(2);420-7.
12) Loesche WJ, Syed SA, Stoll J. In Trypsin like activity in subgingival plaque. A diagnostic marker for Spirochetes and periodontal disease. In: J. of Periodontology 1987; 58: 266-273.
13) Gmur R, Strub JR. Guggenheim B: Prevalence of Bacteroid forsythus and B.gingivalis in subgingival plaque of periodontally treated patients short recall, J. Perio.Res.1989;2(1):113-122. |