Introduction
The base metal alloys like Cobalt-Chromium were introduced into dentistry by H.STELLITE in 1929. These alloys then, were so inexpensive that the new ingots were melted, cast and the surplus metal was either discarded or sold back to the supplier by weight as scrap, even though they were purchased at a very meagre price. When using these inexpensive non-precious alloys for casting procedures, technician used to melt the new ingots rather than mixing the cast metal with new ingots, as it was the practice with precious metal alloys [6].
Casting procedure requires more molten metal to be forced than is needed to fill the mould. The surplus metal separated from the casting is known as "button". The possibility for the "button" to be reused with the addition of 1/3rd new metal to produce an acceptable casting is a matter of economy [10].
Thus, by adding new metal, composition of the alloy that might be altered in the earlier casting procedures may be replenished. This practice is especially important with metal ceramic alloys, which contain trace elements essential for bonding of porcelain. Mclean and Sced [12] suggested that, addition of 1/3rd new metal during recyling would be sufficient to restore the required physical properties [8].
Bonding of porcelain to metal is thought to result from mechanical inter-locking[2], vander waals forces[2] chemical bonding [2],[4],[5] and compressive bonding. All these mechanisms require wetting of the metal surface with porcelain during sintering[9]. The contribution of various bonding mechanisms has been debated, but the chemical bond is considered necessary to achieve the adequate bond strength for metal ceramic restoration.
Experiments and Research regarding matching of the co-efficient of thermal expansion of metal and porcelain and determining the role of metal oxides [1], [2],[3] on the bond strength has, produced successful metal-ceramic restoration.
It has been observed that the "button" or previously cast metal/alloy melts at a higher temperature than that of new ingot due to the inclusion of surface oxide layer. Also it is likely that the previously cast metal may exhibit altered surface chemistry [12] and texture which is vital for producing a bond of adequate strength between metal and porcelain.
These possible alterations in surface chemistry, texture and melting temperature raise the question, whether the recast base metal with and without the addition of 1/3rd new metal would produce similar bond strength. The present study was undertaken with an aim to determine the effect of base metal alloy castings and recasting with one-third of new base metal alloy on bond strength of porcelain in vitro.
Materials And Method
Study location:This study was conducted in the department of Prosthodontics, S.D.M College of Dental Sciences and Hospital, Dharwad, Karnataka.
Preparation:Standardized sprue of 15mm length and a diameter of 2mm was attached to plastic copings. To the sprue, a reservoir of 3mm diameter was attached 2mm away from the plastic copings. On a 3 X crucible former (Whipmix) four of plastic copings were arranged diagonally opposite to each other with an angle of 600 to the horizontal plane. The 3 X casting ring was lined with Kera Vlies (cellulose) liner. These patterns were sprayed with Lubrofilm(DENTAURUM, Germany) to reduce the surface tension.
Mixing and investment:The preproportioned packets of Castorit Super C (150 gms) with the Castorit Super C mixing liquid 34 ml (DENTAURUM, Germany) were mixed, and invested in the 3 X casting ring. After 40 minutes the casting ring was separated from the crucible former and transferred to the cold burnout furnace (KAVO, Germany). The casting ring was placed vertically with the crucible facing downwards to facilitate the free flow of molten wax. The casting ring was preheated to dry at 2500C for 60 minutes, there after it was heated to the final temperature of 9500 C gradually at a rate of 50 C/minute and was heat soaked at 9500 C for 30 minutes.
Casting procedure:Two types porcelain compatible alloys, Nickel-Chromium (Remanium CS, DENTAURUM, Germany) and Cobalt- Chromium (Remanium CD,DENTAURUM Germany) were used for this study. Six pellets of Nickel-Chromium alloy were placed in crucible and introduced into the furnace for preheating for 10 minutes and later cast in the induction casting machine (Degutron, Degussa Germany). Then the casting ring was allowed to cool for 5 minutes and quenched in cold water. The same investing, burnout and casting procedure was repeated for three other rings to have the casting in Nickel-Chromium and four other rings in Cobalt-Chromium alloys to obtain Ist batch of 16 copings each of Nickel -Chromium and Cobalt-Chromium alloys. Four rings were cast with the sand blasted and cleaned buttons, obtained from the first casting, to produce 16 copings of each metal (Nickel-Chromium, and Cobalt-Chromium) consequently which were termed as IInd batch.
The remaining buttons after the first casting were weighted in an electronic weighing machine (Precisa, Switzerland) and approximately 1/3rd of the new metal added for the next batch of casting . The addition of 1/3rd new metal was repeated for both the alloys and cast in the 4 rings for both of the metals to obtain IIIrd batch of 16 copings of each alloy
Retrieved castings were sand blasted with 100 grit of aluminum oxide, placing metal copings vertical to the outlet with 60 psi of compressed air. These metal copings were separated from the button and finished with a Tungsten carbide trimmer. Then each of the specimens were sand blasted with 60 grit of aluminum oxide for 30 seconds and the specimens were subjected to ultrasonic cleaning (BANDELIN SONOREX Super, Germany) for 5 minutes (Plate No.7). Separate containers were used to differentiate among different alloy types and batches. The three batches of Nickel-Chromium and Cobalt-Chromium are designed by alphabets A and B respectively. Thus the copings cast from the new ingots were termed as A-I, from recast of the button as A-II and from recast of the button with the addition of 1/3rd new metal as A-III. The same was repeated for Cobalt-Chromium B-I, B-II and B-III respectively.
It was required to prepare a metal die with a flat base on which the porcelain fused specimens could be tested using the Universal testing machine.. All the 96 metal copings by batch wise were checked for the fit over the metal die.
These Nickel-Chromium and Cobalt-Chromium alloy copings were dried and transferred on to the silicon nitride firing tray (Ivoclar, Leischtenstein) and placed in the porcelain furnace (Ivoclar, Programat P-90, Leischtenstein) for oxidation, with 1400 C. Ten milli-grams of D4 shade opaquer (Duceram of Germany) was dispensed on to the porcelain tile and mixed with 10ml of mixing liquid
These specimens were cooled and a silky layer of D4 shade porcelain opaquer was applied and placed in the furnace. This was gradually fired upto 9250 C for 2 minutes without vacuum. This was cooled gradually to the room temperature, another layer (II layer) of opaquer over the Ist layer was applied over each coping to completely mask the colour of the underlying metal. This was placed back into the furnace and gradually fired up to 9250 C, with vacuum from 6200 to 9250 C and held at that temperature for 2 minutes before releasing the vacuum. These specimens were cooled to the room temperature. 10 mg of D4 shade dentine porcelain powder (body porcelain) was dispensed on a porcelain tile and 10ml of liquid was added to it. This was mixed uniformly to build the coping with the dentine material having the mesio-distal width of 7mm incisally and thickness of 1.1mm approximately and having a height of 11mm cervico-incisally on the labial aspect. This dentine layer was carved to simulate the anatomy of natural tooth and the excess liquid was removed by condensation and placed back into the furnace and fired gradually upto 9200 C with a holding time of 1 minute. It was then allowed to cool gradually to the room temperature. This same method was followed for the remaining 95 copings.
Assessing the bond strength:Later, the specimens were tested on Universal testing machine. They were subjected to analysis at a cross head speed of 0.002 inch/minute in a compressive mode until the separation of the bond. The amount of force required to fracture and separate porcelain from metal was noted for each specimen of all batches of both alloys which formed the basis data for this study. This basic data for each alloy and batch was tabulated and subject to statistical analysis. Descriptive statistics were analyzed and Analysis of Variance was used to compare means between three batches of alloys. Student t test was used to compare bond strengths between two different alloys
Results
Table 1 shows bond strength values in Mega Newton /m2 (kg/mm2 converted to Mega Newton / m2 ) between porcelain and base metal alloy of Nickel-Chromium. Kilograms to Newtons conversion is achieved by the formula N= K x 9.8 /122/106. Ten samples were tested in each of the three batches. Batch A-I consisted of new metal, Batch A-II only the button of new metal and the Batch A-III consisted of the button with the addition of 1/3 of new metal.
 | Table 1: Bond Strength Values Of The Test Samples In Mega Newton/m2
 |
Table 2 shows the Bond strength values between porcelain and base metal alloy of Cobalt-Chromium. Ten samples were tested in three batches. Batch-I consisted of new metal, Batch-II had only the button of new metal and Batch-III consisted of button with the addition of 1/3 of new metal.
 | Table 2: Bond Strength Of The Test Samples In Mega Newton/m2
 |
The mean and standard deviation values of three batches of Nickel-Chromium alloy are shown in Table 3.
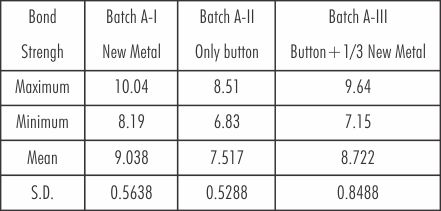 | Table 3: Mean And Standard Deviation Of Three Batches Of Nickel-chromium Alloy In Mega Newton/m2
 |
For the Batch-I (new metal) the mean value was 9.038 and standard deviation, 0.5638. The mean value of Batch-II (only the button) was 7.517 with the standard deviation, 0.5288. The mean value of the Batch-III (button plus the addition of 1/3 new metal) was 8.722 and the standard deviation being 0.8488 showed little variation from that of the Batch-I.
The mean and standard deviation values of bond strength of three batches of Cobalt-Chromium alloy are shown in Table 4.
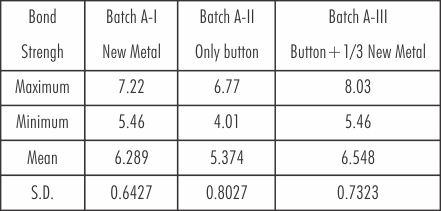 | Table 4: Mean And Standard Deviation Of Three Batches Of Cobalt-chromium Alloy In Mega Newton/m2
 |
Mean value of the bond strength in Batch I (new metal) was 6.289 with the standard deviation of 0.6427. For the Batch-II (only button) the mean value was lower than the Batch I that is 5.374 and the standard deviation, 0.8027. The mean value of the Batch III (button plus the addition of 1/3 new metal) was 6.546 with little variation from that of the Batch I and the standard deviation being 0.7233.
Table 5 shows the statistical comparison between the three batches of Nickel-Chromium alloy using ANOVA (Analysis of Variance) test.
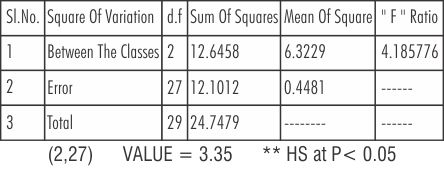 | Table 5: Anova Table Showing The Comparsion Between The Three Batches Of Nickel - Chromium
 |
There was a difference of high significance between the two batches, that is Batch I (new metal) and Batch II (only button); and between the Batch III (button plus the addition of 1/3 new metal) and Batch II (only button). The F-Ratio (Fisher's ratio) was 14. 185776 at(P<0.05).
Table 6 shows the statistical comparison between the three batches of Cobalt-Chromium I Batch (new metal), II Batch (only button) and III batch (button plus the addition of 1/3 new metal) using Anova (Analysis of Variance ) test. The result from the analysis show a difference high significance between the three batches with the F-Ratio(Fisher's ratio) being 7.399 at (P<0.05).
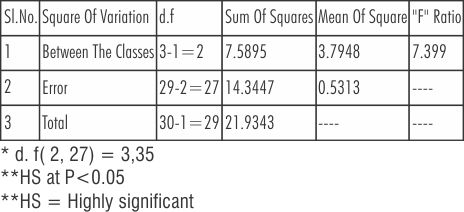 | Table 6: Anova Table Showing The Comparison Between The Three Batches Of Cobalt - Chromium
 |
Table 7 shows that 't'-value (paired student's 't' test) between the three batches of Nickel-Chromium Batch I (new metal) and Batch II (only button) was highly significant, (t=7.6 at P<0.05), similarly the 't' value between the Batch II and Batch III (button plus addition of 1/3rd new metal) was 4.01 which was highly significant at (P<0.5). No significant difference was noticed between the Batch I (new metal) and Batch III (button plus the addition 1/3rd new metal as the 't' value was 1.59 at (P>0.05).
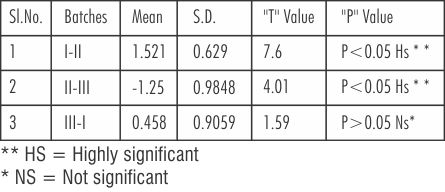 | Table 7 : ‘t'-value Between The Three Batches Of Nickel-Chromium
 |
Table 8 shows that 't' value (paired) between the three batches of Cobalt- Chromium Batch I (new metal) and Batch II (only button) the value was 2.56, significant at (P<0.05). Similarly the 't'-value between the Batch II (only button) and Batch III (button plus the addition of 1/3 new metal) was 4.48, significant at (P<0.05) while there was no significant difference between the Batch I (new metal) and Batch III ( new metal plus the addition of 1/3 new metal) of Cobalt Chromium, that is the 't'-value was less, 0.73 at (P>0.05).
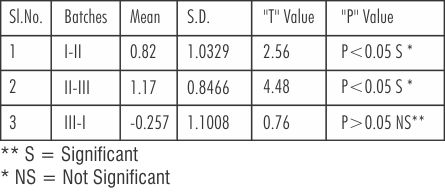 | Table 8: 't' Value Between The Three Batches Of Cobalt-Chromium
 |
Table 9 shows that 't' value (unpaired student's 't'-test) between the three batches of Nickel Chromium and Cobalt-Chromium.
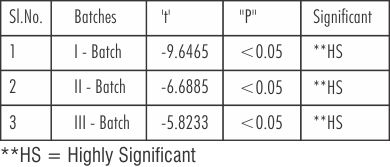 | Table 9: Showing 't' Value (Unpaired) Between The Three Batches Of Nickel-Chromium And Cobalt-Chromium
 |
The 't' value between the Batch I of A & B (new metal) of Nickel-Chromium and Cobalt- Chromium was -9.6465; significant at (P<0.05), suggesting the higher bond strength values of Nickel significance, t = -6.6885 at (P<0.05), between the Batch II of A &B suggesting the higher bond strength values of Nickel Chromium again Cobalt Chromium the Batch III of A & B (button plus addition 1/3 new metal ) also, there was high significance between Nickel - Chromium and Cobalt-Chromium, t -5.8233 at (P<0.05). These results also conclude that the bond strength values of Nickel- Chromium over the Cobalt-Chromium were higher.
Discussion
Though, there was no significant change reported in physical properties on recasting up to four generations[6] , from the results of this study it appeared that the bond strength values between Nickel-Chromium and Cobalt-Chromium with porcelain were higher than the values obtained from the recast metal which could be of potential clinical importance, that is for Nickel-Chromium alloy the mean bond strength value of Batch-I (new metal) was 9.038 (SD=0.5638) and that of the Batch-II (only button) was less, that is a mean value of 7.517 with (SD=0.5288). Similarly for Cobalt-Chromium alloy the mean bond strength value of Batch-I (new metal) was 6.289 (SD=0.6427) and that of the Batch-II (only button) was less, that is the mean value being 5.374 with (SD=0.8027). These results indicate the reduction in the bond strength at the interface for the recast metal (only button) for both the base metal alloys. This could be attributed to more metallic oxide dispersion at the ceramo metal interface[1], as well as the thickness of the micro porosities at the internal oxidation zone which increase as the metal is recast without the addition of 1/3rd new metal/alloy[8].
In concurrent to this, Moffa et al [11] claimed that the presence of oxide layer augmented bonding by contributing to compressive bonding forces as postulated by Vickery and Bandenelly (1968). The decease in the bond strength could also be explained by the depletion of few essential trace elements which oxide readily during the initial casting procedure.
Donald et al indicated that the procedure of adding varying amounts of new metal to the old is not necessary claiming that the physical properties are not changed when cast for four generations [6].
Proof to the contrary noticed in the present study when the “Buttons” were used for casting Batch II of alloy A & B showed less bond strength with porcelain as compared to Batch I of A &B (table 3&4). The difference in mean value of bond strength between Batch I & II of both alloys was statistically significant as per Table 7 & 8. Therefore it is recommended that only button should not be used for recasting. However when 1/3rd new metal was added to the button, the bond strength was not significantly affected. The evaluation of results showed that the mean bond strength of Batch III (button plus the addition of 1/3 new metal) of Nickel-Chromium alloy was 8.722 (SD=0.8488) showing little variation from the mean bond strength value of Batch I (new metal), that is (t=1.59 and P>0.05 i.e. not significant). Similar was the case with Cobalt-Chromium alloy, where the mean bond strength of the Batch III (button plus the addition of 1/3 new metal) was 6.546 (SD=0.7323) again showing little variation from the Batch I (new metal), that is (t=0.73 and P>0.05 i.e. not significant). These results suggest that the strength of the bond at the interface can be restored with the addition of 1/3 new metal to the once cast metal. From the literature available it is understood that the components of the alloy lost/altered in the earlier castings might have been replenished after the addition of 1/3 new metal thus maintaining the physical property of bond strength.
Though in 1986 Donald thought that the recasting with the addition of new metal was not cost effective considering the cost of the alloy a decade ago, compared to the present economic situation. However, in Third world countries like India the factors like labour and time are not included in consideration.
Due to devaluation of currency, changes in duties and other taxation the recycling of metal is an economic solution to reduce the cost factor particularly if the required properties are not unduely altered. In the present situation, recycling of costlier ceramo metal alloy with the addition of 1/3 new metal effectively proves the point.
Summary And Conclusion
In this project it was observed that the addition of 1/3rd new metal to the alloy once cast, restores the bonding Characteristics of porcelain fused to metal and also that Nickel-Chromium alloy has superior bonding properties with porcelain than Cobalt-Chromium alloy.
Hence, before definitive recommendation can be made for the recycling of an alloy with the addition of 1/3rd new metal, further research is necessary to determine the nature and extent of alteration in the chemical composition of the alloy at the interface between metal and porcelain on a larger sample size.
The values of bond strength of porcelain with various groups and batches of two commercially available base metal alloys are pertained to the materials, their nature and fabrication as well as testing methodology employed in the present study were technique sensitive. The values may not be repeated if the materials and methodology differs.
Reference
1. Anusavice K.J., Ringle R.D, Fairhurst C.W. Bonding mechanism in a ceramic non-precious alloy system. J.Biomed.Master.Research
2. Bagby M., Marshall S.J, Marshall G.W. Metal ceramic Compatibility. A review of literature J.Proshet.Dent.1990;63(1):21
3. Baran G.R.The metallurgy of Ni-Cr alloys for fixed Prosthodotics. J.Prosthet.Dent.1983;50:639
4. Bert Cotti R.L.Porcelain to metal Bonding and Compatibility In Mclean J.W.ed. Dental Ceramics.First International Symposium on Ceramics. Chicago ; Quintessence 1983:415
5. Caitucoli F. Influence of the degree of Oxidation of the alloy on the bond strength of the connection between meal and ceramic material Rev.Odonto.Stomatolmidi fr.1972;30;120
6. Denton l.smith. Dental casting alloys : technical and economic considerationsin U.S.A. Int.Dent.J.1991:33(1):25.
7. Edward F.Leone. Carl W.Fairhurst. Bond strength and mechanical Properties of dental porcelain enamels. J.Proshet Dent.1967;Aug:155.
8. Heiner Weber. The clinical acceptance of Dental Nickel-Chromium alloys Int.Dent.Journal.1982 ;31(1) : 49.
9. Jau-Min Hong, Michel E.Razzoog, Brien R.Lang. The effect of recasting on the oxidation layer of a palladium-silver Porcelain alloy. J.Prosthet.Dent.1988; 59(4):420
10. John W.Mclean. The nature of dental ceramics and their chemical use. Quin. Publishing Co.1979
11. King B.W. Duck worth W.H. Nature of adherence of porcelain -enamels to metals. .J.Am.Ceram, Soc.1959:42:504
12. Sced I. R. Mclean J.W. The strength of metal ceramic bonds with base metals containing chromium. Brit.Dent. J.1972:132:232. |