Introduction
The greatest challenge in clinical research is development of bioactive surgical additives, which help to regulate inflammation and increase the speed of healing process.1 Platelets isolated from the peripheral blood are an autologous source of growth factors i.e. growth factors stored in the alpha granules of platelets include platelet derived growth factor, insulin like growth factor, vascular endothelial growth factor and transforming growth factor beta2 which are able to stimulate cell proliferation, matrix remodeling and angiogenesis.
These growth factors are released by activation of the platelets by substances or stimuli such as thrombin, calcium chloride, collagen or adenosine 5c - diphosphate.3 Platelet concentrates were originally used in transfusion medicine for the treatment and prevention of hemorrhage due to severe thrombopenia, which is often caused by medullar aplasia,acute leukemia or significant blood loss during surgeries which are long lasting.4 The use of platelet concentrates to improve healing and to replace fibrin glues, as first described by Whitman et al5 has been explored considerably during the last decade.
Classification of platelet concentrates
Several techniques for platelet concentrates have been devised, however confusion exists as each method leads to a different product with different biology and potential uses.For all practical reasons these concentrates can be classified into four categories.6
P-PRP - Pure Platelet Rich Plasma
L-PRP - Leucocyte and Platelet Rich Plasma
P-PRF - Pure Platelet Rich Fibrin
L-PRF - Leucocyte and Platelet Rich Fibrin
To understand the concept a set of three parameters must be defined which are as follows:
A) Preparation kits and centrifuge used:
A1) Size of the centrifuge
A2) Duration of the procedure
A3) Cost of the device and kits
A4) Theergonomy of the kit and the complexity of the procedure.
Size of the centrifuge could be heavy(cumbersome) or light (compact). A compact centrifuge would be a preferable choice for clinical applications in dentistry. Duration of the procedure couldbequick (<20 min.), long (20 - 60 min.), very long (>1 hr.).Cost of the device and repeated cost of reagents and kits are also an important parameter. Automated systems were developed for the sole reason of ergonomics. Parameters (A) define the practical characteristics of each technique.
B) Contents of the concentrates:These parameters define the basic pharmacological relevance of the product and lead to indications for potential application.
B1) Final volume of the concentrate (depends on the initial blood harvest).
B2) Efficiency in collecting the platelets.
B3) Efficiency in collecting leucocytes.
B4) Preservation of these contents during the process.
C) Relates to fibrin network that supports the platelet and leucocyte concentrate during its application
C1) Density depends on the fibrinogen during preparation.7
C2) Fibrin Polymerization type
Most protocols lead to low density fibrin gel which can be utilized for surgical application but lack a true fibrin support matrix. On the contrary, a highdensityfibrin network means that, not only the platelet concentrates act as a biomaterial but also the matrix itself has healing effects.8
Fibrinogen is activated by thrombin, which initiates polymerization into fibrin. Two distinct biochemical architectures of fibrin fibrillae can be observed:9
A) Condensed Tetramolecular or bilateral junctions
B) Connected Trimolecular or equilateral junctions
From a clinical perspective the bilateral junctions result due to high thrombin concentrations, which leads to a dense network of monofibers similar to a fibrin glue. This type is least favorable to cytokine enmeshment and cellular migration. On the other hand a slow physiological fibrin polymerization results in higher percentage of equilateral junctions, leading to a flexible network capable of cytokine entrapmentand cellular migration.9
Based on the above mention classification each category of concentrates will be discussed in relation to the protocol used.
LEUCOCYTE POOR or PURE PLATELET - RICH PLASMA (P-PRP)
Pure platelet concentrates for topical use were first developed as an application for the classical transfusion platelet units and were first reported for maxillofacial surgery.3 The P-PRP can be obtained by automated or manual methods.
Automated Protocol:
1) PLASMAPHERESIS: The first method of producing the platelet concentrates for topical use was known as plasmapheresis, which was a cell separator resulting in differential ultracentrifugation (3000g). Different blood components,such as platelets, leucocytes and RBC's were first separated from platelet poor plasma which was then re-infused in the patient.10 Despite the sophisticated equipment used, the final PRP always contained residual RBC's and leucocyte and it was a cumbersome process which required the help of haemotologist.
2) VIVOSTAT PRF CENTRIFUGE (VIVOLUTION, Denmark): This is a advanced cell separator and was designed to produce the vivostat fibrin sealent. It produces a leucocyte poor platelet concentrate for surgical use with the help of a specific preparation kit. The drawbacks include only a few publications studies,cumbersome and very expensive procedure and damage to the platelets occur during the process.11
Manual Protocol
1) ANITUA's PRGF (Plasma rich in growth factors): In 1999,Anitua first described plasma rich in growth factors12 or Preparation rich in growth factors.13 Subsequently it was commercialized by BTI (Bio technology institute,Victoria Spain). The protocol includes the collection of venous blood that is centrifuged in several small test tubes for 8 minutes at 460g. After the centrifugation cycle typically three layers are seen in the test tube. The top most part in the test tube contains Plasma poor in growth factors (PPGF) which is discarded with help of pipetting, at this point care should be taken to avoid turbulence, remaining plasma i.e. (PRGF) is collected with a pipette,using 'eyeballing' as measuring tool. (The act of eyeballing is to measure or weigh something without any tools). 10% calcium chloride solution is added to induce fibrin polymerization. An unstable PRGF gel is obtaining after 15 mins, which has to be used immediately.
Some inconsistencies in the PRGF protocol exist e.g In the original description of the protocolmost of the plasma (after discarding a small fraction as described above) was collected, including the 'buffy coat' layer that contains most of the platelets and leucocytes but in later applications of this method14,15 the authors claim that the buffy coat layer was not collected. The objective of this approach was to avoid the collection of leucocytes, but it seems to be technically imprecise and in danger of yielding irreproducible results. Moreover, it also leads toa low platelet collection. Efficiency because platelets and leucocytes are found together in the intermediate layer after lowspin centrifugation.16 Although it is an inexpensive method but lacks the ergonomy and reproducibility.
2) NAHITA's PRP: is similar to Anituas method.17
LEUCOCYTE- AND PLATELET RICH PLASMA (L-PRP)
Manual Protocol
CURASAN METHOD (Germany): 10 Most commonly used method. The blood sample is drawn into a citrated tube. The sample tube is then spun in a standard centrifuge for 10 minutes at 2400 rpm to produce PPP. The PPP and buffy coat is taken up into a syringe with a long cannula and an additional air-intake cannula. A second centrifugation(15 minutes at 3600 rpm) is performed to concentrate the platelets. The second supernatant is also taken up by along cannula and an air-intake cannula. For each 8 mL of blood, the volume of supernatant is about 0.6-0.7 mL; this is the PRP, to be used for then surgical procedure. Just before the time of the application,the PRP is combined with an equal volume of a sterile saline solution containing 10% calcium chloride (a citrate inhibitor that allows the plasma to coagulate) and 100 U/mL of sterile bovine thrombin (an activator that allows polymerization of the fibrin into an insoluble gel, which causes the platelets to degranulate and release theindicated mediators and cytokines); the result should be asticky gel that will be relatively easy to apply to the surgicaldefects. The PPP can be stored for use as a protective barrier over the wound.18,19,20
Recent publications have indicated that PRP prepared from 8 to 10 mL of whole blood is sufficient for periodontal regenerative therapies.21 However, in oral and maxillofacial reconstruction, 8 to 500 mL of whole blood should be drawn, so as to obtain the greater amounts of PRP needed for larger surgical defects.3,22
FRIADENT SCHUTZE (Austria) : 23 Uses similar protocol as described above.
REGEN (Switzerland) : Regen method uses a separator gel within the centrifugation tubes with the aim of improving the collection of platelets and leucocytes.
PLATELTEX (Slovakia): The Plateltex protocol uses specific gelifying agents, such as calcium gluconate and lyophilized purified batroxobin, which is an enzyme that cleaves fibrinopeptide. This brings about fibrin polymerization without bovine thrombin and resultant gelling in about 10 minutes.24
The drawbacks of these techniques are that these protocols require substantial manual procedures, meaning that the preparation process is time consuming, and only lead to small volumes of L-PRP.
Apart from the above-mentioned drawbacks adapted kits can be quite expensive if used frequently and the final product exhibits a low-density fibrin matrix, which is strong enough for application as fibrin glue but quickly dissolves. The reproducibility of the end product is questionable that is in case the buffy coat layer is not completely collected,the platelet collection efficiency decreases and a PPRP can sometimes be obtained instead of LPRP. (Figure 1)
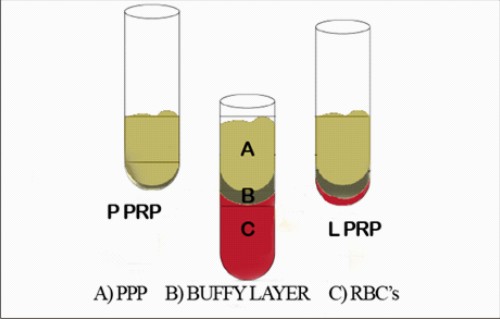 | Figure 1.Classical manual platelet-rich plasma (PRP) protocol using a two-step centrifugation procedure .Three layers are obtainedafter first centrifuge cycle: red blood cells (RBCs), 'buffy coat' (BC) layer and platelet-poor plasma (PPP).
 |
Automated Protocol
PCCS(Platelet Concentrate Collection System): was developed by 3I
Citrated whole blood is transferred into the first compartment and centrifuged for a short period to obtain the three layers RBC, buffy coat, PPP.Then, by opening of a tubule and using air pressure, thesuperficial layers (i.e. PPP and buffy coat) are transferred to the second chamber and centrifuged again but for a longer period. At the final step using the same air pressure system,most of the PPP layer is transferred back into the first compartment and thus discarded. Finally product is leucocyte rich and has similar characteristics to the manual Curasan PRP describedin the beginning.
Published reports25-27 point out to the fact that these systems (Curasan and PCCS) have greater ease of handling and shorter preparation times than the SmartPReP and Tisseel systems.
SMARTPReP SYSTEM
The two-chamber device automatically transfers the top layers(PPP and buffy coat) into the second chamber based on variations in weight and centrifugation speed. SmartPReP is a multifunction system,21 using a specific collection and separation kit. The centrifuge usedin this system can also be used to concentrate stem cells from bone marrow aspirates.
It has the advantage of being an autologous system and hence there is no risk of disease transmission. SmartPReP system produces PRP gel as well as fibrin glue. Furthermore, this system has larger blood containers for centrifugation that enables the operator to obtain 90 to 180 mL of whole blood, leading to sufficientamount of PRP for maxillofacial or plastic and reconstructive surgical procedures.
MAGELLAN APS SYSTEM
The Magellan APS (Autologous Platelet Separator) is anadvance cell separator with optical reader. This device is compact and designed for small blood samples of up to 50 mL. Platelet collection efficiency is high, but cell preservation is not known.The company claims that the leucocyte content is also high.28
GPS (Gravitational Platelet Separation System)
This system uses a two-chamber centrifugation device with two-step centrifugation protocol.29 The main difference is that the PPP is discarded after the first centrifugation using a syringe and tubules, and the second centrifugation step is performed with the RBC layer. The final PRP concentrate is collected by aspiration of the buffy coat layer on the surface of the RBC base. The procedure is thus inversed, but the final result seems to be similar. The main drawbacks of all these techniques are that they require expensive and cumbersome centrifuges and collection/preparation kits, the final concentrates dissolve quickly, similar to fibrin glue. Their use in daily practice remains uncommon and itis no longer available.
LEUCOCYTE-POOR OR PURE PLATELET-RICH FIBRIN (P-PRF)
Fibrinet PRFM kit is the only system under this category
The system comprises of two tubes, one for blood collection and another forPRFM clotting, together with a transfer device. A small amount of blood (9 mL) is drawn into a collectiontube, which contains tri-sodium citrate as an anticoagulantand a proprietary separator gel, which is then centrifuged for sixminutes at high speed.
Typically three layers in the order of RBCs,buffy coat and PPP are obtained. Buffy coat and PPP areeasily transferred to a second tube containing CaCl2 with the help of a specifically designed tube connection system.The clotting process is triggered by the presence of CaCl2 and the tube is immediately centrifuged for 15 min, after which a stable PRFM clot can be collected. It is claimed by the company that the system produces a 'natural' platelet concentrate owing to the absence of bovine thrombin. However, this claim is doubtful because the blood is mixed with anticoagulant and separation gel, leading to what could be considered unnatural conditions.
This protocol is similar to L-PRP protocols, such as the Curasan method. The main difference is that only very low amounts of leucocytes are collected owing to the specific separator gel used in the method.
LEUCOCYTE - AND PLATELET-RICH FIBRIN (L-PRF) OR CHOUKROUN'S PRF
Was developed in France by Choukroun et al.30 It can be considered as a second-generation platelet concentratebecauseit is produced without any anticoagulants or gelifying agents.1 Venous blood iscollected in dry glass tubes(without anticoagulants) and centrifuged at low speed 3000rpm at about 400g for 12min(Process protocol, Nice, France).31 PRF can be considered as an autologous healing biomaterial, incorporating in a matrix of autologous fibrin most leukocytes, platelets and growth factors harvested from a simple blood sample.32,33(Fig 2)
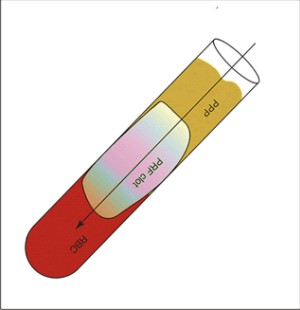 | Figure 2.Choukroun'sPRF : PPP at the top layer, PRF clot in the middle and RBC's in the bottom layer
 |
In the absence ofanticoagulants, platelet activation and fibrin polymerization are triggeredin a natural manner immediately. After centrifugation,three layers are formed: the RBC layer at the base, acellular plasmalayerat the top and a PRF clot in the middle.
The PRF clot forms leads to formation of a strong fibrin matrix with a complex three-dimensional architecture, in which most ofthe platelets and leucocytes from the harvested blood are concentrated.The PRF clot becomes a strong membrane, when pressed between to sterile gauze pieces. Applications of this autologous biomaterial have been describedin oral,34 maxillofacial,35 ENT (ear, nose, throat) 36 and plastic surgery.37
Conclusion
The advent of second generation platelet concentrates i.e. Choukroun's PRF has overcome the drawbacks encountered in the PRP protocol. The Choukroun's protocol allows the production of a high quantity of L-PRF clots using either a specific centrifuge that takes eight tubes or any modified laboratory centrifuge, making it possible to produce even more clots for larger surgeries. Additional advantage of this method is its low cost and the simplicity of the procedure, which allowsnatural means, that is, without the use of chemicals or unnatural conditions. Therefore, this method seems to be most suitable for widespread use in daily practice and is actually the main technique in some countries, including France, Italy and Israel. An accurate working knowledge of the biomaterial, its biology, efficiency and limits are necessary to optimize its use in daily practice for a widespread use.
References
1. Dohan DM, Choukroun J, DissA,Dohan SL, DohanAJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 2006;101: e37-e44.
2. Toffler M, Toscano N, Holtzclaw D, Del Corso M, EhrenfestDohan D. Introducing Choukroun's Platelet Rich Fibrin (PRF) to the reconstructive surgery Milieu. The Journal of Implant and Advanced Clinical Dentistry 2009; 1: 21-32.
3. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, GeorgeffKR.Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral MedOralPathol Oral RadiolEndod. 1998; 85: 638-646.
4. Sunitha R, Munirathnam N. Platelet rich fibrin: Evolution of a second generation platelet concentrate. Indian J Dent Res 2008;19:42-46.
5. Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery.J Oral Maxillofac Surg. 1997;55:1294-9.
6. DohanEhrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet rich plasma (P-PRP) to leucocyte and platelet - rich fibrin (L-PRF).Trends Biotechnical 2009; 27:158- 167.
7. Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features offibrinogen and fibrin.Ann N Y Acad Sci. 2001; 936:11-30.
8. Clark, R.A. Fibrin and wound healing. Ann N Y Acad. Sci.2001;936: 355-367.
9. Van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis.Ann N Y Acad Sci. 2001; 936: 426-37.
10. Weibrich G, Kleis WK, Hafner G, Hitzler WE, Wagner W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank.Clin Oral Implants Res. 2003;14:357-62.
11. Leitner GC, Gruber R, Neumüller J, Wagner A, Kloimstein P, Höcker P, Körmöczi GF, BuchtaC.Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems.Vox Sang. 2006; 91:135-9.
12. Anitua, E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants.Int J Oral Maxillofac Implants. 1999;14:529-35.
13. Anitua E, Sánchez M, Orive G, Andía I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials2007;28: 4551-4560.
14. Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, Andia I.Effectivenessof autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers.J Biomed Mater Res B ApplBiomater. 2008;84:415-21.
15. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I.Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245-51.
16. Weibrich G, Kleis WK, Hitzler WE, Hafner G.Comparison of the platelet concentrate collection system with the plasma-rich-in-growth-factors kit to produce platelet-rich plasma: a technical report. Int J Oral Maxillofac Implants. 2005;20:118-23.
17. Tamimi FM, Montalvo S, Tresguerres I, Blanco Jerez L. A comparative study of two methods for obtaining platelet-rich plasma.J Oral Maxillofac Surg. 2007;65:1084-93.
18. Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res2002; 37:300-6.
19. Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral, and guided tissue regeneration versus platelet-rich plasma and bovine porous bone mineral in the treatment of intrabonydefects: a reentry study. J Periodontol2002; 73:198-205.
20. Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. PlastReconstrSurg2001; 107:229 -37.
21. Weibrich G, Kleis WK, Kunz-Kostomanolakis M, Loos AH, Wagner W. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants2001;16:693-9.
22. Gonshor A. Technique for producing platelet-rich plasma and plateletconcentrate: background and process. Int J Periodontics Restorative Dent 2002; 22:547-57.
23. Weibrich G, Kleis WK, Buch R, Hitzler WE, HafnerG.The Harvest Smart PRePTM system versus the Friadent-Schütze platelet-rich plasma kit.Clin Oral Implants Res. 2003;14:233-9.
24. Mazzucco L, Balbo V, Cattana E, BorziniP.Platelet-rich plasma and platelet gel preparation using Plateltex. Vox Sang. 2008;94:202-8.
25. Weibrich G, Kleis WK. Curasan PRP kit vs. PCCS PRP system. Collection efficiency and platelet counts of 2 different methods for the preparation of platelet-rich plasma. Clin Oral Implants Res 2002;13:437-43.
26. Appel TR, Pötzsch B, Müller J, von Lindern JJ, Berge SJ, Reich RH. Comparison of three different preparations of platelet concentrates for growth factor enrichment. Clin Oral Implants Res2002; 13:522-8.
27. Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants 2002;17:184-90.
28. Christensen K, Vang S, Brady C, Isler J, Allen K, Anderson J, Holt D.Autologous platelet gel: an invitro analysis of platelet-rich plasma using multiple cycles. J Extra Corpor Technol. 2006; 38:249-53.
29. Marlovits S, Mousavi M, Gäbler C, Erdös J, Vécsei V.A new simplified technique for producing platelet-rich plasma: a short technical note. Eur Spine J. 2004; 13:S102-6.
30. Choukroun J, Adda F, Schoeffler C, Vervelle A. Uneopportunité en paro-implantologie: le PRF.Implantodontie 2001; 42:55-62. (French).
31. Dohan DM, Del Corso M, Charrier JB. Cytotoxicityanalyses of Choukroun's platelet-rich fibrin (PRF)on a wide range of human cells: The answer toa commercial controversy. Oral Surg Oral MedOral Pathol Oral RadiolEndod 2007; 103:587-593.
32. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin(PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. OralSurg Oral Med Oral Pathol Oral RadiolEndod. 2006; 101: e45-50.
33. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF):a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for plateletconcentrates? Oral Surg Oral Med Oral Pathol Oral RadiolEndod 2006; 101: e51-55.
34. Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2006;101:e56-60.
35. Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a secondgeneration platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift.OralSurg Oral Med Oral Pathol Oral RadiolEndod. 2006;101:299-303.
36. Diss A, Dohan DM, Mouhyi J, Mahler P.Osteotome sinus floor elevation using Choukroun'splatelet-rich fibrin as grafting material: a one-year prospective pilotstudy with microthreaded implants. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2008; 105:572-9.
37. Choukroun JI, Braccini F, Diss A, Giordano G, Doglioli P, Dohan DM Influence of platelet rich fibrin (PRF) onproliferation of human preadipocytes and tympanic keratinocytes: anew opportunity in facial lipostructure (Coleman's technique) andtympanoplasty? Rev LaryngolOtolRhinol (Bord). 2007;128:27-32. |