Introduction
The placement of orthodontic appliances on teeth not only impedes the maintenance of a proper oral hygiene1, 2 but also increases the level of cariogenic bacteria in the oral cavity3-5, leading to serious biofilm-related side-effects such as white spot lesions and gingival inflammation6-8, compromising facial esthetics after an often lengthy and costly course of orthodontic treatment.The most common site for bacterial adhesion and biofilm formation is at the bracket-adhesive-enamel junction, an area that is difficult to clean by daily brushing8, 9. Oral biofilms at this junction not only cause damage to oral hard and soft tissues but also weaken the bond strength of adhesives10-12. Excessive adhesive around brackets especially provide a site for the rapid adhesion and growth of bacteria13. Furthermore, the surface of an orthodontic adhesive is often rough, with a gap of around 10um at the adhesive enamel interface due to polymerization shrinkage. This provides adhering bacteria with a protected site against oral cleansing forces14, 15. Consequently, the bracket-adhesive-enamel junction is a critical site for bacterial adhesion and biofilm formation in orthodontic patients.
Composition and Mechanism of Biofilm Formation
Oral biofilms, including orthodontic biofilms (oral biofilms formed on orthodonticbiomaterials during active orthodontic treatment or retention phase), are diversecommunities of microorganisms on dental hard and soft tissues and dental biomaterials. These biofilms are embedded in an extracellular matrix of polymers of host and microbial origin, possessing complex spatial, heterogeneous and dynamic structures16. Oral biofilms in general comprise about 80% water and 20% of solid phase components including proteins, carbohydrates, fat, and inorganic components. The composition of orthodontic biofilms varies during the course of treatment. Placement of an orthodontic appliance increases not only the amount of biofilm,but also the prevalence of cariogenic bacteria such as mutans streptococci and lactobacilli17
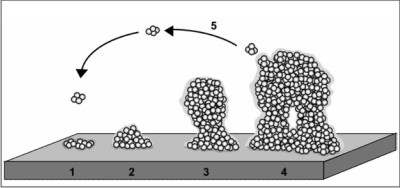 | Fig. The development of a biofilm, depicted as a five-stage process. Stage 1: initial attachment of cells to the surface; stage 2: production of the extracellularexopolysaccharide matrix; stage 3: early development of biofilm architecture; stage 4: matura
 |
Molecules are adsorbed to the tooth surface within seconds immediately after cleaning or following initial exposure to the oral environment, and remain functional(53). These molecules are derived mainly from saliva, but, in the subgingival region, molecules originate from gingival crevicular fluid. The conditioning film alters the properties of the surface, and bacteria interact directly with the constituent molecules.
Stoodley et al 18 described biofilm formation through sequential steps in which the initial attachment of planktonic bacteria to a solid surface is followed by their subsequent proliferation and accumulation in multilayer cell clusters, and the final formationof the bacterial community enclosed in a self-produced polymeric matrix. Once the structure has developed, some bacteria are released into the liquid medium, enabling the biofilm to spread over the surface.
Factors influencing orthodontic biofilm formation
Banding vs bonding Bandinginduced more orthodontic biofilm formation ,gingival inflammation and white spot lesions than bonding19. Most biofilm was located at the gingival margin, with more band surface being covered by biofilm at the supragingival area than at the sub-gingival one20.
Adhesives: Excessive composite resin at the bracket-enamel-adhesive junction is prone to bacterial adhesion, especially since polymerization shrinkage may yield a gap with a width of up to 10 um at the adhesive-enamel interface where bacteria find themselves protected against oral cleansing forces21. Roughness of the composite surface predisposes to rapid attachmentand growth of oral micro-organisms (Weitmannand Eames, 1975; Gwinnett and Ceen, 1979).
Brackets, Elastics and Springs
According to thermodynamic rules, bacteria with high surface-free energy prefer high surface-free energy materials (Busscheret al., 1984; Van Dijket al., 1987).It has been suggested that metal brackets increase bacterial adhesion because oftheir high surface energy compared with that of plastic and ceramic brackets (Eliadeset al., 1995). Therefore, it might be expected that streptococci adherepreferentially to metal brackets, which have higher surface-free energy (Weerkampet al., 1985; Kilianet al. Invivo, maxillary brackets harvested more S. mutansand S. sobrinusthan mandibular brackets22 while labial brackets harvested more biofilm than lingual brackets23 Brackets have shown the most adsorption capability of whole saliva protein constituents while intra oral elastics and springs have shown much less affinity to salivary proteins.
Method of ligation
The labialenamel of teeth ligated with an elastomeric ringmay exhibit a significantly higher number ofmicro-organisms in the plaque than incisorsligated with steel wire (Forsberg et al., 1991)5. Clinical observation has indicated that acommon site of demineralization is at thejunction between the bonding resin and theenamel, just peripheral and commonly gingivalto the bracket base (Gwinnett and Ceen, 1979).
Arch Wires
Complicated appliance designs with loops and auxiliary arch wirescreate areas that are difficult to clean and may therefore enhance biofilm formation24.
Retainers
Removable orthodontic retainers may attract oral biofilm andpresent new retention sites, similar to removable acrylic plates, favoring bacterialadhesion and growth25. Fixed retainers are in direct contact with the enamel surface and cannot be removed for extensive cleaning like removable ones. Therefore they are generallyconsidered to yield increased biofilm formation with negative consequences with respect to gingival inflammation
Thus, it is conceivable that different types of biofilms will be formed on those orthodontic surfaces as they are of constructed from various materials, their elasticity and their topography varies.
Prevention of orthodontic Biofilm
Development of orthodontic materials attracting less biofilms has been a goal fordecades. Attempts have been made to develop effective antimicrobial adhesives toprevent orthodontic biofilms.
Mechanical Control
Effective manual or powered brushing and the use ofinterdental brushes is still by far the most important measure for oral hygienecontrol in orthodontic patients .The auxiliary interdental brush is helpful in removing biofilm formation behind the wire during orthodontic treatment26. Despite the fact that new designs of general toothbrushes came on the market, longer brushing time and proper brushing techniques are still necessary for good oral hygiene in orthodontic patients.
Chemical Biofilm Control
A variety of chemical biofilm control measuresincluding incorporation of antimicrobials in toothpastes, mouthrinses, varnishes and adhesives are currently used by the dental profession, including orthodontists. Chlorhexidine however, still remains the most effective antimicrobial inreducing biofilm-induced iatrogenic side effects in orthodontic patients and S.mutanslevels . Unfortunately, long-term use of chlorhexidine is known to stainteeth and tongue and affect taste sensation. The benefits of fluoride containing toothpastes and mouthrinses in preventing caries have been well establishedand besides aiding enamel remineralization, fluoride acts as a buffer to neutralizeacids produced by bacteria and suppresses their growth.
Modification of Orthodontic Materials
Modification of orthodontic materials iseither aimed at reducing the consequences of orthodontic biofilms or at preventingbiofilm formation and includes incorporation of chemicals in the adhesive or coating of bracket and wire materials
Clinical implications and future research
It has been shown that surface roughness increases the bacterial adhesion forces, it would be desirable that orthodontists minimize the adhesive surface roughness by smoothing, polishing, or varnishing after bonding. This is a simple yet efficient way to reduce bacterial adhesion at the bracket-adhesive enamel junction. Orthodontic material manufacturers might also provide additional procedures to decrease the surface roughness of their products for clinical practice.Although the hydrophobicities of stainless steel, adhesives, and enamel were different, the salivary conditioning film decreased this difference significantly and there with also the bacterial adhesion forces. This indicates that the development of antibacterial modification of orthodontic materials should always take the effects of a salivary conditioning film into account. As the adhesion forces of initial colonizers were significantly stronger than those of the more cariogenic strains, while adhesion of initial colonizers is determinant for the strength of adhesion of the overlaying biofilm structure65, future research should be directed toward prevention of the adhesion of initial colonizers.The long duration of orthodontic treatments and salivary flow in the oral cavity favor orthodontic materials with non-leaching, long lasting bactericidal properties. The modification of an orthodontic adhesive with a quaternary ammonium compound provided efficient contact-killing, with promising prospects for clinical application. Future research to enhance the mechanical strength by improving the processing conditions, i.e. curing the samples at a higher temperature, or adding a diacrylate to increase the density of crosslinking, would be approaches worth exploring.
References
1. Effectiveness of a chlorhexidine dentifricein orthodontic patients: a randomized-controlled trial. J ClinPeriodontol 2006;33:421-426.
2. Ogaard B, Ten Bosch JJ. Regression of white spot enamel lesions. A new optical method for quantitative longitudinal evaluation in vivo. Am J Orthod Dentofacial Orthop 1994;106:238-242.
3. Pender N. Aspects of oral health in orthodontic patients. Br J Orthod 1986;13:95-103.
4. Mattingly JA, Sauer GJ, Yancey JM, Arnold RR. Enhancement of Streptococcus mutans colonization by direct bonded orthodontic appliances. J Dent Res 1983;62:1209-1211.
5. Forsberg CM, Brattstrom V, Malmberg E, Nord CE. Ligature wires and elastomeric rings:two methods of ligation, and their association with microbial colonization of Streptococcus mutansand lactobacilli. Eur J Orthod 1991;13:416-420.
6. Ogaard B. White spot lesions during orthodontic treatment: mechanisms and fluoride preventive aspects Seminars in Orthodontics 2008;14:183-193.
7. Willmot D. White spot lesions after orthodontic treatment. Seminars in Orthodontics 2008;14:209-219.
8. Mei L, Busscher HJ, Van der Mei HC, Chen Y, De Vries J, Ren Y. Oral bacterial adhesion forces to biomaterial surfaces constituting the bracket-adhesive-enamel junction in orthodontic treatment. Eur J Oral Sci 2009;117:419-426.
9. Sukontapatipark W, El-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod 2001;23:475-484.
10. Guzman-Armstrong S, Chalmers J, Warren JJ. Ask us. White spot lesions: prevention and treatment. Am J Orthod Dentofacial Orthop 2010;138:690-696.
11. Petersilka GJ. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontol 2000 2011;55:124-142.
12. Matasa CG. Microbial attack of orthodontic adhesives. Am J Orthod Dentofacial Orthop 1995;108:132-141.
13. Holmen L, Thylstrup A, Artun J. Surface changes during the arrest of active enamel carious lesions in vivo. A scanning electron microscope study.ActaOdontolScand 1987;45:383-390.
14. Ahn HB, Ahn SJ, Lee SJ, Kim TW, Nahm DS. Analysis of surface roughness andsurface free energy characteristics of various orthodontic materials. Am J Orthod DentofacialOrthop 2009;136:668-674
15. Lee SP, Lee SJ, Lim BS, Ahn SJ. Surface characteristics of orthodontic materials andtheir effects on adhesion of mutans streptococci. Angle Orthod 2009;79:353-360.
16. Al Mulla AH, Kharsa SA, Kjellberg H, Birkhed D. Caries risk profiles in orthodontic patients at follow-up using Cariogram. Angle Orthod 2009;79:323-330.
17. Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000 2010;55:16-35
18. Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol 2002;56:187-209.
19. Boyd RL, Baumrind S. Periodontal considerations in the use of bonds or bands on molars in adolescents and adults. Angle Orthod 1992;62:117-126.
20. Demling A, Demling C, Schwestka-Polly R, Stiesch M, Heuer W. Influence of lingual orthodontic therapy on microbial parameters and periodontal status in adults. Eur J Orthod 2009;31:638-642.
21. Sukontapatipark W, El-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod 2001;23:475-484.
22. Ahn SJ, Lim BS, Lee SJ. Prevalence of cariogenic streptococci on incisor brackets detected by polymerase chain reaction. Am J Orthod Dentofacial Orthop 2007;131:736-741.
23. Van der Veen MH, Attin R, Schwestka-Polly R, Wiechmann D. Caries outcomes after orthodontic treatment with fixed appliances: do lingual brackets make a difference? Eur J Oral Sci 2010;118:298-303.
24. Ogaard B. White spot lesions during orthodontic treatment: mechanisms and fluoride preventive aspects Seminars in Orthodontics 2008;14:183-193.
25. Batoni G, Pardini M, Giannotti A, Ota F, Giuca MR, Gabriele M et al. Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur J Oral Sci 2001;109:388-392.
26. Ahn SJ, Lim BS, Lee YK, Nahm DS. Quantitative determination of adhesion patterns of cariogenic streptococci to various orthodontic adhesives. Angle Orthod 2006;76:869-875. |